COVID-19 Vaccination Breakthrough Infections in a Real-World Setting: Using Community Reporters to Evaluate Vaccine Effectiveness
- PMID: 36090603
- PMCID: PMC9451035
- DOI: 10.2147/IDR.S373183
COVID-19 Vaccination Breakthrough Infections in a Real-World Setting: Using Community Reporters to Evaluate Vaccine Effectiveness
Erratum in
-
Erratum: COVID-19 Vaccination Breakthrough Infections in a Real-World Setting: Using Community Reporters to Evaluate Vaccine Effectiveness [Corrigendum].Infect Drug Resist. 2022 Sep 20;15:5543-5544. doi: 10.2147/IDR.S389192. eCollection 2022. Infect Drug Resist. 2022. PMID: 36164336 Free PMC article.
Abstract
Purpose: Coronavirus disease 2019 (COVID-19) has highlighted the need for new methods of pharmacovigilance. Here, we use adult community volunteers to obtain systematic information on vaccine effectiveness and the nature and severity of breakthrough infections.
Methods: Between December 15, 2020 and September 16, 2021, 11,826 unpaid community-based volunteers reported the following information to an on-line registry: COVID-19 test results, vaccination (Pfizer, Moderna, or Johnson & Johnson) and COVID-19 symptoms. COVID-19 infections were described based on vaccination status at the time of infection: 1) fully vaccinated, 2) partially vaccinated (received first of two-dose vaccines or were <14 days post-final dose), or 3) unvaccinated.
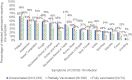
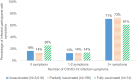
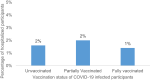
Results: Among 8554 participants who received any COVID-19 vaccine, COVID-19 infections were reported by 74 (1.0%) of those who were fully vaccinated and 198 (2.3%) of those who were partially vaccinated at the time of infection. Among the 74 participants who reported a breakthrough infection after full vaccination, the median time from vaccination to reported positive test result was 104.5 days (interquartile range: 77-135 days), with no difference among vaccine manufacturers. One quarter (25.7%) of breakthrough infections in the fully vaccinated cases were asymptomatic and most (>97%) fully vaccinated participants reported no symptoms or only mild symptoms compared to 89.3% of the unvaccinated cases. Only 1.4% of fully vaccinated participants reported experiencing at least 3 moderate-to-severe symptoms compared to 7.8% in the unvaccinated.
Conclusion: Person-generated health data, also referred to as patient-reported outcomes, is a useful approach for quantifying breakthrough infections and their severity and for comparing vaccines.
Trial registration: Clinicaltrials.gov NCT04368065, EU PAS Register EUPAS36240.
Keywords: COVID-19; SARS-CoV-2; breakthrough infections; patient-reported outcomes; symptoms; vaccines.
© 2022 Reynolds et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Choi A, Ibanez LI, Strohmeier S, Krammer F, Garcia-Sastre A, Schotsaert M. Non-sterilizing, infection-permissive vaccination with inactivated influenza virus vaccine reshapes subsequent virus infection-induced protective heterosubtypic immunity from cellular to humoral cross-reactive immune responses. Front Immunol. 2020;11:1166. doi:10.3389/fimmu.2020.01166 - DOI - PMC - PubMed
-
- U.S. Food and Drug Administration. Guideline for Postmarketing Reporting of Adverse Drug Experiences. Rockville, MD: US Food and Drug Administration; 1992.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

