Dual-functional composite scaffolds for inhibiting infection and promoting bone regeneration
- PMID: 36090611
- PMCID: PMC9449864
- DOI: 10.1016/j.mtbio.2022.100409
Dual-functional composite scaffolds for inhibiting infection and promoting bone regeneration
Abstract
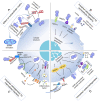
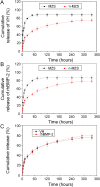
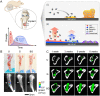
The treatment of infected bone defects is an intractable problem in orthopedics. It comprises two critical parts, namely that of infection control and bone defect repair. According to these two core tasks during treatment, the ideal approach of simultaneously controlling infection and repairing bone defects is promising treatment strategy. Several engineered biomaterials and drug delivery systems with dual functions of anti-bacterial action and ostogenesis-promotion have been developed and demonstrated excellent therapeutic effects. Compared with the conventional treatment method, the dual-functional composite scaffold can provide one-stage treatment avoiding multiple surgeries, thereby remarkably simplifying the treatment process and reducing the treatment time, overcoming the disadvantages of conventional bone transplantation. In this review, the impaired bone repair ability and its specific mechanisms in the microenvironment of pathogen infection and excessive inflammation were analyzed, providing a theoretical basis for the treatment of infectious bone defects. Furthermore, we discussed the composite dual-functional scaffold composed of a combination of antibacterial and osteogenic material. Finally, a series of advanced drug delivery systems with antibacterial and bone-promoting capabilities were summarized and discussed. This review provides a comprehensive understanding for the microenvironment of infectious bone defects and leading-edge design strategies for the antibacterial and bone-promoting dual-function scaffold, thus providing clinically significant treatment methods for infectious bone defects.
Keywords: Antibacterial; Bone repair; Dual-functional scaffold; Infectious bone defect.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
Dual-functional Hydroxyapatite scaffolds for bone regeneration and precision drug delivery.J Mech Behav Biomed Mater. 2024 Sep;157:106661. doi: 10.1016/j.jmbbm.2024.106661. Epub 2024 Jul 14. J Mech Behav Biomed Mater. 2024. PMID: 39018918 Review.
-
Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models.Acta Biomater. 2018 Oct 1;79:265-275. doi: 10.1016/j.actbio.2018.08.015. Epub 2018 Aug 18. Acta Biomater. 2018. PMID: 30125670
-
Chitosan-Based Biomaterial Scaffolds for the Repair of Infected Bone Defects.Front Bioeng Biotechnol. 2022 May 4;10:899760. doi: 10.3389/fbioe.2022.899760. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35600891 Free PMC article. Review.
-
Dual-functional thermosensitive hydrogel for reducing infection and enhancing bone regeneration in infected bone defects.Mater Today Bio. 2024 Jan 20;25:100972. doi: 10.1016/j.mtbio.2024.100972. eCollection 2024 Apr. Mater Today Bio. 2024. PMID: 38312799 Free PMC article.
-
MicroRNA-loaded biomaterials for osteogenesis.Front Bioeng Biotechnol. 2022 Sep 19;10:952670. doi: 10.3389/fbioe.2022.952670. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36199361 Free PMC article. Review.
Cited by
-
Biomaterial-Based Scaffolds as Carriers of Topical Antimicrobials for Bone Infection Prophylaxis.Pol J Microbiol. 2025 Jun 18;74(2):232-243. doi: 10.33073/pjm-2025-019. eCollection 2025 Jun 1. Pol J Microbiol. 2025. PMID: 40544521 Free PMC article. Review.
-
Bioactive VS4-based sonosensitizer for robust chemodynamic, sonodynamic and osteogenic therapy of infected bone defects.J Nanobiotechnology. 2024 Jan 16;22(1):31. doi: 10.1186/s12951-023-02283-6. J Nanobiotechnology. 2024. PMID: 38229126 Free PMC article.
-
From an Antimicrobial Agent to a constituent of 3D Printed Heterogenous Scaffolds Stimulating Bone Characteristics: An In-vitro and Animal model evaluation.Regen Ther. 2025 Aug 12;30:558-574. doi: 10.1016/j.reth.2025.08.001. eCollection 2025 Dec. Regen Ther. 2025. PMID: 40837861 Free PMC article.
-
3D printing technology and its combination with nanotechnology in bone tissue engineering.Biomed Eng Lett. 2024 Jan 30;14(3):451-464. doi: 10.1007/s13534-024-00350-x. eCollection 2024 May. Biomed Eng Lett. 2024. PMID: 38645590 Free PMC article. Review.
-
Programmed Transformation of Osteogenesis Microenvironment by a Multifunctional Hydrogel to Enhance Repair of Infectious Bone Defects.Adv Sci (Weinh). 2025 Mar;12(10):e2409683. doi: 10.1002/advs.202409683. Epub 2025 Jan 22. Adv Sci (Weinh). 2025. PMID: 39840502 Free PMC article.
References
-
- Baker C.E., Moore-Lotridge S.N., Hysong A.A., Posey S.L., Robinette J.P., Blum D.M., Benvenuti M.A., Cole H.A., Egawa S., Okawa A., Saito M., McCarthy J.R., Nyman J.S., Yuasa M., Schoenecker J.G. Bone fracture acute phase response-A unifying theory of fracture repair: clinical and scientific implications. Clin. Rev. Bone Miner. Metabol. 2018;16(4):142–158. - PMC - PubMed
-
- Bai J., Wang H., Gao W., Liang F., Wang Z., Zhou Y., Lan X., Chen X., Cai N., Huang W., Tang Y. Melt electrohydrodynamic 3D printed poly (epsilon-caprolactone)/polyethylene glycol/roxithromycin scaffold as a potential anti-infective implant in bone repair. Int. J. Pharm. 2020;576 - PubMed
-
- Hassani Besheli N., Mottaghitalab F., Eslami M., Gholami M., Kundu S.C., Kaplan D.L., Farokhi M. Sustainable release of vancomycin from silk fibroin nanoparticles for treating severe bone infection in rat tibia osteomyelitis model. ACS Appl. Mater. Interfaces. 2017;9(6):5128–5138. - PubMed
-
- Afewerki S., Bassous N., Harb S., Palo-Nieto C., Ruiz-Esparza G.U., Marciano F.R., Webster T.J., Furtado A.S.A., Lobo A.O. Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopaedic applications. Nanomedicine. 2020;24 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

