Palmitoylethanolamide attenuates neurodevelopmental delay and early hippocampal damage following perinatal asphyxia in rats
- PMID: 36090655
- PMCID: PMC9452789
- DOI: 10.3389/fnbeh.2022.953157
Palmitoylethanolamide attenuates neurodevelopmental delay and early hippocampal damage following perinatal asphyxia in rats
Erratum in
-
Corrigendum: Palmitoylethanolamide attenuates neurodevelopmental delay and early hippocampal damage following perinatal asphyxia in rats.Front Behav Neurosci. 2022 Dec 19;16:1115398. doi: 10.3389/fnbeh.2022.1115398. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36600990 Free PMC article.
Abstract
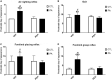
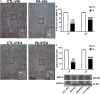
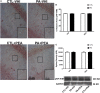
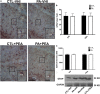
Impaired gas exchange close to labor causes perinatal asphyxia (PA), a neurodevelopmental impairment factor. Palmitoylethanolamide (PEA) proved neuroprotective in experimental brain injury and neurodegeneration models. This study aimed to evaluate PEA effects on the immature-brain, i.e., early neuroprotection by PEA in an experimental PA paradigm. Newborn rats were placed in a 37°C water bath for 19 min to induce PA. PEA 10 mg/kg, s.c., was administered within the first hour of life. Neurobehavioral responses were assessed from postnatal day 1 (P1) to postnatal day 21 (P21), recording the day of appearance of several reflexes and neurological signs. Hippocampal CA1 area ultrastructure was examined using electron microscopy. Microtubule-associated protein 2 (MAP-2), phosphorylated high and medium molecular weight neurofilaments (pNF H/M), and glial fibrillary acidic protein (GFAP) were assessed using immunohistochemistry and Western blot at P21. Over the first 3 weeks of life, PA rats showed late gait, negative geotaxis and eye-opening onset, and delayed appearance of air-righting, auditory startle, sensory eyelid, forelimb placing, and grasp reflexes. On P21, the hippocampal CA1 area showed signs of neuronal degeneration and MAP-2 deficit. PEA treatment reduced PA-induced hippocampal damage and normalized the time of appearance of gait, air-righting, placing, and grasp reflexes. The outcome of this study might prove useful in designing intervention strategies to reduce early neurodevelopmental delay following PA.
Keywords: PEA; hippocampal CA1 area; neurodevelopmental disorder (NDD); neuroprotection; palmitoylethanolamide; perinatal asphyxia; reflexes.
Copyright © 2022 Herrera, Udovin, Kobiec, Toro-Urrego, Kusnier, Kölliker-Frers, Luaces, Otero-Losada and Capani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Adcock L. M., Papile L. A. (2008). “Perinatal asphyxia,” in Manual of neonatal care, eds Cloherty J. P., Eichenwald E. C., Stark A. R. (Philadelphia, PA: Lippincott, Williams, and Wilkins; ), 518–528.
-
- Ahmad A., Crupi R., Impellizzeri D., Campolo M., Marino A., Esposito E., et al. (2012). Administration of palmitoylethanolamide (PEA) protects the neurovascular unit and reduces secondary injury after traumatic brain injury in mice. Brain Behav. Immun. 26 1310–1321. 10.1016/j.bbi.2012.07.021 - DOI - PubMed
-
- Azzopardi D., Robertson N. J., Bainbridge A., Cady E., Charles-Edwards G., Deierl A., et al. (2016). Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): a proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 15 145–153. 10.1016/S1474-4422(15)00347-6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

