Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: The CaReMe CKD study
- PMID: 36090671
- PMCID: PMC9459126
- DOI: 10.1016/j.lanepe.2022.100438
Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: The CaReMe CKD study
Abstract
Background: Digital healthcare systems data could provide insights into the global prevalence of chronic kidney disease (CKD). We designed the CaReMe CKD study to estimate the prevalence, key clinical adverse outcomes and costs of CKD across 11 countries.
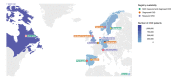
Methods: Individual-level data of a cohort of 2·4 million contemporaneous CKD patients was obtained from digital healthcare systems in participating countries using a pre-specified common protocol; summarized using random effects meta-analysis. CKD and its stages were defined in accordance with current Kidney Disease: Improving Global Outcomes (KDIGO) criteria. CKD was defined by laboratory values or by a diagnosis code.
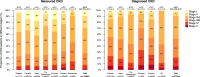
Findings: The pooled prevalence of possible CKD was 10·0% (95% confidence interval 8.5‒11.4; mean pooled age 75, 53% women, 38% diabetes, 60% using renin-angiotensin-aldosterone system inhibitors). Two out of three CKD patients identified by laboratory criteria did not have a corresponding CKD-specific diagnostic code. Among CKD patients identified by laboratory values, the majority (42%) were in KDIGO stage 3A; and this fraction was fairly consistent across countries. The share with CKD based on urine albumin-creatinine ratio (UACR) alone (KDIGO stages one and two) was 29%, with a substantial heterogeneity between countries. Adverse events were common; 6·5% were hospitalized for CKD or heart failure, and 6·2% died, annually. Costs for renal events and heart failure were consistently higher than costs for atherosclerotic events in CKD patients across all countries.
Interpretation: We estimate that CKD is present in one out of ten adults. These individuals experience significant adverse outcomes with associated costs. The prevalence of CKD is underestimated when using diagnostic codes alone. There is considerable public health potential in diagnosing CKD and providing treatments to those currently undiagnosed.
Funding: The study was sponsored by AstraZeneca.
Keywords: Chronic kidney disease; Costs; Epidemiology; Global health; Outcomes; Prevalence; Primary care; Public health; Renal impairment.
© 2022 The Author(s).
Conflict of interest statement
J.S. reports stock ownership in Anagram kommunikation AB and Symptoms Europe AB. J.B. holds a full-time position at AstraZeneca as an epidemiologist. A.B. reports no competing interests. M.G.V. has received personal fees from Amgen, Vifor, FMC, Otsuka, Medice, AstraZeneca. Grants received from Amgen, FMC, Dutch Kidney Foundation, European communion, Health Holland (Ministery of Economic affairs). Non-financial support received from FMC, Calcison, NedMag. P.B.M. reports lecture fees and travel to meetings support from Vifor, Astrazeneca, Pharmacomsos, Napp, Astellas, lecture fees from Novartis, Astellas and grants from Boehringer Ingelheim outside the submitted work. A.K. has received research grants and speaking honoraria from Astrazeneca, Novonordisk and Boehringer Ingelheim. T. T. G. declares speaker and consulting fees from AstraZeneca, BIAL, Daiichi-Sankyo, MSD, Medinfar and Novartis. TTG holds shares in MTG. M.B. has received honoraria from Astra Zeneca, Janssen, Lilly, Boehringer Ingelheim, Sanofi, Amgen and Novo Nordisk. K.I.B. has received grants to his institution from AstraZeneca for this study and for lectures and consulting from Novo Nordisk, Sanofi, Lilly, Boehringer Ingelheim and Merck Sharp & Dohme. M.T. holds a full-time position by an independent statistical consultant company, Statisticon AB, Uppsala, Sweden, of which AstraZeneca Nordic is a client. L.J. reports no competing interests. M.S. declares a speaker fee from AstraZeneca. G.V.P reports no competing interests. N.T. reports grants and personal fees from AstraZeneca, grants and personal fees from Janssen, grants and personal fees from BI-Lilly, grants and personal fees from Otsuka, grants, personal fees and other from Tricida, personal fees and other from Pulsedata, personal fees and other from Mesentech, personal fees and other from Renibus, other from ClinPredict, outside the submitted work; In addition, N.T. has a patent for A microfluidic device for point of care detection of urine albumin pending.
Figures


References
-
- Chronic Kidney Disease Basics | Chronic Kidney Disease Initiative | CDC. published online Sept 13. https://www.cdc.gov/kidneydisease/basics.html. 2021. Accessed 23 November 2021.
-
- Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23:S163–S172. - PubMed
LinkOut - more resources
Full Text Sources

