Enhancing arginase 2 expression using target site blockers as a strategy to modulate macrophage phenotype
- PMID: 36090747
- PMCID: PMC9424864
- DOI: 10.1016/j.omtn.2022.08.004
Enhancing arginase 2 expression using target site blockers as a strategy to modulate macrophage phenotype
Abstract
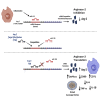
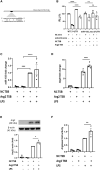
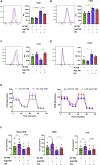
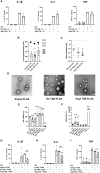
Macrophages are plastic cells playing a crucial role in innate immunity. While fundamental in responding to infections, when persistently maintained in a pro-inflammatory state they can initiate and sustain inflammatory diseases. Therefore, a strategy that reprograms pro-inflammatory macrophages toward an anti-inflammatory phenotype could hold therapeutic potential in that context. We have recently shown that arginase 2 (Arg2), a mitochondrial enzyme involved in arginine metabolism, promotes the resolution of inflammation in macrophages and it is targeted by miR-155. Here, we designed and tested a target site blocker (TSB) that specifically interferes and blocks the interaction between miR-155 and Arg2 mRNA, leading to Arg2 increased expression and activity. In bone marrow-derived macrophages transfected with Arg2 TSB (in the presence or absence of the pro-inflammatory stimulus LPS), we observed an overall shift of the polarization status of macrophages toward an anti-inflammatory phenotype, as shown by significant changes in surface markers (CD80 and CD71), metabolic parameters (mitochondrial oxidative phosphorylation) and cytokines secretion (IL-1β, IL-6, and TNF). Moreover, in an in vivo model of LPS-induced acute inflammation, intraperitoneal administration of Arg2 TSB led to an overall decrease in systemic levels of pro-inflammatory cytokines. Overall, this proof-of-concept strategy represent a promising approach to modulating macrophage phenotype.
Keywords: MT: non-coding RNAs; PLGA; arginase 2; macrophages; miR-155; microRNAs; target site blocker; transfection.
© 2022 The Author(s).
Conflict of interest statement
The authors have no relevant conflicts of interest to declare other than the receipt of funding form the named agencies to carry out the current work.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

