Bioengineering the Vascularized Endocrine Pancreas: A Fine-Tuned Interplay Between Vascularization, Extracellular-Matrix-Based Scaffold Architecture, and Insulin-Producing Cells
- PMID: 36090775
- PMCID: PMC9452644
- DOI: 10.3389/ti.2022.10555
Bioengineering the Vascularized Endocrine Pancreas: A Fine-Tuned Interplay Between Vascularization, Extracellular-Matrix-Based Scaffold Architecture, and Insulin-Producing Cells
Abstract
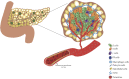
Intrahepatic islet transplantation is a promising β-cell replacement strategy for the treatment of type 1 diabetes. Instant blood-mediated inflammatory reactions, acute inflammatory storm, and graft revascularization delay limit islet engraftment in the peri-transplant phase, hampering the success rate of the procedure. Growing evidence has demonstrated that islet engraftment efficiency may take advantage of several bioengineering approaches aimed to recreate both vascular and endocrine compartments either ex vivo or in vivo. To this end, endocrine pancreas bioengineering is an emerging field in β-cell replacement, which might provide endocrine cells with all the building blocks (vascularization, ECM composition, or micro/macro-architecture) useful for their successful engraftment and function in vivo. Studies on reshaping either the endocrine cellular composition or the islet microenvironment have been largely performed, focusing on a single building block element, without, however, grasping that their synergistic effect is indispensable for correct endocrine function. Herein, the review focuses on the minimum building blocks that an ideal vascularized endocrine scaffold should have to resemble the endocrine niche architecture, composition, and function to foster functional connections between the vascular and endocrine compartments. Additionally, this review highlights the possibility of designing bioengineered scaffolds integrating alternative endocrine sources to overcome donor organ shortages and the possibility of combining novel immune-preserving strategies for long-term graft function.
Keywords: 3D- bioprinting; alternative endocrine sources; beta cell replacement; bioengineering; biomaterials; extracellular matrix; type 1 diabetes; vascularized endocrine pancreas.
Copyright © 2022 Pignatelli, Campo, Neroni, Piemonti and Citro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Bioengineering of a human iPSC-derived vascularized endocrine pancreas for type 1 diabetes.Cell Rep Med. 2025 Feb 18;6(2):101938. doi: 10.1016/j.xcrm.2025.101938. Epub 2025 Feb 7. Cell Rep Med. 2025. PMID: 39922198 Free PMC article.
-
Recreating the Endocrine Niche: Advances in Bioengineering the Pancreas.Artif Organs. 2025 Apr;49(4):541-555. doi: 10.1111/aor.14950. Epub 2025 Jan 23. Artif Organs. 2025. PMID: 39844747 Review.
-
Bioengineering a highly vascularized matrix for the ectopic transplantation of islets.Islets. 2013 Sep-Dec;5(5):216-25. doi: 10.4161/isl.27175. Epub 2013 Nov 21. Islets. 2013. PMID: 24262950 Free PMC article.
-
Directed self-assembly of a xenogeneic vascularized endocrine pancreas for type 1 diabetes.Nat Commun. 2023 Feb 16;14(1):878. doi: 10.1038/s41467-023-36582-1. Nat Commun. 2023. PMID: 36797282 Free PMC article.
-
Can We Re-Engineer the Endocrine Pancreas?Curr Diab Rep. 2018 Oct 2;18(11):122. doi: 10.1007/s11892-018-1072-7. Curr Diab Rep. 2018. PMID: 30280279 Review.
Cited by
-
Clinical Translation and Implementation of a Bioartificial Pancreas Therapy: A Qualitative Study Exploring the Perspectives of People With Type 1 Diabetes.Transplant Direct. 2024 Sep 25;10(10):e1711. doi: 10.1097/TXD.0000000000001711. eCollection 2024 Oct. Transplant Direct. 2024. PMID: 39328250 Free PMC article.
-
Bioengineering of a human iPSC-derived vascularized endocrine pancreas for type 1 diabetes.Cell Rep Med. 2025 Feb 18;6(2):101938. doi: 10.1016/j.xcrm.2025.101938. Epub 2025 Feb 7. Cell Rep Med. 2025. PMID: 39922198 Free PMC article.
-
Allo Beta Cell transplantation: specific features, unanswered questions, and immunological challenge.Front Immunol. 2023 Nov 23;14:1323439. doi: 10.3389/fimmu.2023.1323439. eCollection 2023. Front Immunol. 2023. PMID: 38077372 Free PMC article. Review.
-
ESOT Roadmap for Advanced Therapy Medicinal Products in Transplantation: Navigating Regulatory Challenges to Enhance Access and Care.Transpl Int. 2024 Oct 14;37:13485. doi: 10.3389/ti.2024.13485. eCollection 2024. Transpl Int. 2024. PMID: 39469665 Free PMC article. Review.
-
3D evaluation of the extracellular matrix of hypoxic pancreatic islets using light sheet fluorescence microscopy.Islets. 2024 Dec 31;16(1):2298518. doi: 10.1080/19382014.2023.2298518. Epub 2024 Jan 24. Islets. 2024. PMID: 38267218 Free PMC article.
References
-
- Lablanche S, Vantyghem MC, Kessler L, Wojtusciszyn A, Borot S, Thivolet C, et al. Islet Transplantation versus Insulin Therapy in Patients with Type 1 Diabetes with Severe Hypoglycaemia or Poorly Controlled Glycaemia after Kidney Transplantation (TRIMECO): a Multicentre, Randomised Controlled Trial. Lancet Diabetes Endocrinol (2018) 6:527–37. 10.1016/S2213-8587(18)30078-0 - DOI - PubMed
-
- Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, et al. National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes (2016) 65:3418–28. 10.2337/db16-0234 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

