Strategies to Improve the Safety of iPSC-Derived β Cells for β Cell Replacement in Diabetes
- PMID: 36090777
- PMCID: PMC9448870
- DOI: 10.3389/ti.2022.10575
Strategies to Improve the Safety of iPSC-Derived β Cells for β Cell Replacement in Diabetes
Abstract
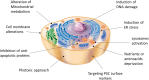
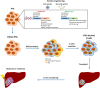
Allogeneic islet transplantation allows for the re-establishment of glycemic control with the possibility of insulin independence, but is severely limited by the scarcity of organ donors. However, a new source of insulin-producing cells could enable the widespread use of cell therapy for diabetes treatment. Recent breakthroughs in stem cell biology, particularly pluripotent stem cell (PSC) techniques, have highlighted the therapeutic potential of stem cells in regenerative medicine. An understanding of the stages that regulate β cell development has led to the establishment of protocols for PSC differentiation into β cells, and PSC-derived β cells are appearing in the first pioneering clinical trials. However, the safety of the final product prior to implantation remains crucial. Although PSC differentiate into functional β cells in vitro, not all cells complete differentiation, and a fraction remain undifferentiated and at risk of teratoma formation upon transplantation. A single case of stem cell-derived tumors may set the field back years. Thus, this review discusses four approaches to increase the safety of PSC-derived β cells: reprogramming of somatic cells into induced PSC, selection of pure differentiated pancreatic cells, depletion of contaminant PSC in the final cell product, and control or destruction of tumorigenic cells with engineered suicide genes.
Keywords: beta cells; cell therapy; induced pluripotent stem cells; safety; type 1 diabetes mellitus.
Copyright © 2022 Pellegrini, Zamarian and Sordi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

