Reduced mitochondrial size in hippocampus and psychiatric behavioral changes in the mutant mice with homologous mutation of Timm8a1-I23fs49X
- PMID: 36090790
- PMCID: PMC9453755
- DOI: 10.3389/fncel.2022.972964
Reduced mitochondrial size in hippocampus and psychiatric behavioral changes in the mutant mice with homologous mutation of Timm8a1-I23fs49X
Abstract
Background: Deafness-dystonia-optic neuronopathy (DDON) syndrome, a condition that predominantly affects males, is caused by mutations in translocase of mitochondrial inner membrane 8A (TIMM8A)/deafness dystonia protein 1 (DDP1) gene and characterized by progressive deafness coupled with other neurological abnormalities. In a previous study, we demonstrated the phenotype of male mice carrying the hemizygous mutation of Timm8a1-I23fs49X. In a follow-up to that study, this study aimed to observe the behavioral changes in the female mutant (MUT) mice with homologous mutation of Timm8a1 and to elucidate the underlying mechanism for the behavioral changes.
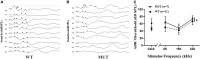
Materials and methods: Histological analysis, transmission electron microscopy (EM), Western blotting, hearing measurement by auditory brainstem response (ABR), and behavioral observation were compared between the MUT mice and wild-type (WT) littermates.
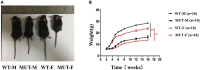
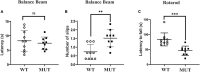
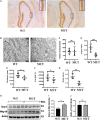
Results: The weight of the female MUT mice was less than that of the WT mice. Among MUT mice, both male and female mice showed hearing impairment, anxiety-like behavior by the elevated plus maze test, and cognitive deficit by the Morris water maze test. Furthermore, the female MUT mice exhibited coordination problems in the balance beam test. Although the general neuronal loss was not found in the hippocampus of the MUT genotype, EM assessment indicated that the mitochondrial size showing as aspect ratio and form factor in the hippocampus of the MUT strain was significantly reduced compared to that in the WT genotype. More importantly, this phenomenon was correlated with the upregulation of translation of mitochondrial fission process protein 1(Mtfp1)/mitochondrial 18 kDa protein (Mtp18), a key fission factor that is a positive regulator of mitochondrial fission and mitochondrial size. Interestingly, significant reductions in the size of the uterus and ovaries were noted in the female MUT mice, which contributed to significantly lower fertility in the MUT mice.
Conclusion: Together, a homologous mutation in the Timm8a1 gene caused the hearing impairment and psychiatric behavioral changes in the MUT mice; the latter phenotype might be related to a reduction in mitochondrial size regulated by MTP18.
Keywords: DDP1; MTFP1; TIMM8A; deafness-dystonia-optic neuronopathy (DDON) syndrome; mitochondrial fission.
Copyright © 2022 Ouattara, Chen, Huang, Chen, Song, Xiao, Li, Guan, Li, Jiang, Xu, Pan and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Frameshift mutation of Timm8a1 gene in mouse leads to an abnormal mitochondrial structure in the brain, correlating with hearing and memory impairment.J Med Genet. 2021 Sep;58(9):619-627. doi: 10.1136/jmedgenet-2020-106925. Epub 2020 Aug 20. J Med Genet. 2021. PMID: 32820032
-
Functional analysis of a novel mutation in the TIMM8A gene that causes deafness-dystonia-optic neuronopathy syndrome.Mol Genet Genomic Med. 2020 Mar;8(3):e1121. doi: 10.1002/mgg3.1121. Epub 2020 Jan 5. Mol Genet Genomic Med. 2020. PMID: 31903733 Free PMC article.
-
Clinical and molecular findings in a patient with a novel mutation in the deafness-dystonia peptide (DDP1) gene.Brain. 2003 Aug;126(Pt 8):1814-20. doi: 10.1093/brain/awg174. Epub 2003 Jun 4. Brain. 2003. PMID: 12805099 Review.
-
Human deafness dystonia syndrome is caused by a defect in assembly of the DDP1/TIMM8a-TIMM13 complex.Hum Mol Genet. 2002 Mar 1;11(5):477-86. doi: 10.1093/hmg/11.5.477. Hum Mol Genet. 2002. PMID: 11875042
-
Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review.
Cited by
-
CHCHD2 rescues the mitochondrial dysfunction in iPSC-derived neurons from patient with Mohr-Tranebjaerg syndrome.Cell Death Dis. 2025 Mar 12;16(1):173. doi: 10.1038/s41419-025-07472-9. Cell Death Dis. 2025. PMID: 40075073 Free PMC article.
-
Neural oscillations in the nucleus accumbens-dorsal hippocampal circuits and behavioral effects of acute fluoxetine administration during the Tail suspension test in mice.Exp Brain Res. 2025 Jun 11;243(7):174. doi: 10.1007/s00221-025-07115-7. Exp Brain Res. 2025. PMID: 40498382
References
-
- Aiken C. E., Tarry-Adkins J. L., Penfold N. C., Dearden L., Ozanne S. E. (2016). Decreased ovarian reserve, dysregulation of mitochondrial biogenesis, and increased lipid peroxidation in female mouse offspring exposed to an obesogenic maternal diet. FASEB J. 30 1548–1556. 10.1096/fj.15-280800 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

