Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review
- PMID: 36091023
- PMCID: PMC9449804
- DOI: 10.3389/fimmu.2022.940562
Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review
Abstract
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant is currently the dominant variant globally. This third interim analysis of a living systematic review summarizes evidence on the effectiveness of the coronavirus disease 2019 (COVID-19) vaccine (vaccine effectiveness, VE) and duration of protection against Omicron.
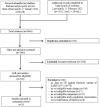
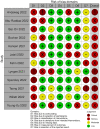
Methods: We systematically searched literature on COVID-19 for controlled studies, evaluating the effectiveness of COVID-19 vaccines approved in the European Union up to 14/01/2022, complemented by hand searches of websites and metasearch engines up to 11/02/2022. We considered the following comparisons: full primary immunization vs. no vaccination, booster immunization vs. no vaccination, and booster vs. full primary immunization. VE against any confirmed SARS-CoV-2 infection, symptomatic, and severe COVID-19 (i.e., COVID-19-related hospitalization, ICU admission, or death) was indicated, providing estimate ranges. Meta-analysis was not performed due to high study heterogeneity. The risk of bias was assessed with ROBINS-I, and the certainty of the evidence was evaluated using GRADE.
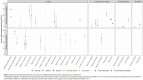
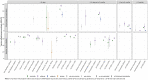
Results: We identified 26 studies, including 430 to 2.2 million participants, which evaluated VE estimates against infections with the SARS-CoV-2 Omicron variant. VE against any confirmed SARS-CoV-2 infection ranged between 0-62% after full primary immunization and between 34-66% after a booster dose compared to no vaccination. VE range for booster vs. full primary immunization was 34-54.6%. After full primary immunization VE against symptomatic COVID-19 ranged between 6-76%. After booster immunization VE ranged between 3-84% compared to no vaccination and between 56-69% compared to full primary immunization. VE against severe COVID-19 ranged between 3-84% after full primary immunization and between 12-100% after booster immunization compared to no vaccination, and 100% (95% CI 71.4-100) compared to full primary immunization (data from only one study). VE was characterized by a moderate to strong decline within 3-6 months for SARS-CoV-2 infections and symptomatic COVID-19. Against severe COVID-19, protection remained robust for at least up to 6 months. Waning immunity was more profound after primary than booster immunization. The risk of bias was moderate to critical across studies and outcomes. GRADE certainty was very low for all outcomes.
Conclusions: Under the Omicron variant, the effectiveness of EU-licensed COVID-19 vaccines in preventing any SARS-CoV-2 infection is low and only short-lasting after full primary immunization, but can be improved by booster vaccination. VE against severe COVID-19 remains high and is long-lasting, especially after receiving the booster vaccination.
Keywords: COVID-19; Omicron variant; SARS-CoV-2; systematic review; vaccination; vaccine effectiveness; variant of concern.
Copyright © 2022 Külper-Schiek, Piechotta, Pilic, Batke, Dreveton, Geurts, Koch, Köppe, Treskova, Vygen-Bonnet, Waize, Wichmann and Harder.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- World Health Organization . Weekly epidemiological update on COVID-19 - 22 march 2022 [Online] (2022). Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on... (Accessed March 28, 2022).
-
- Harder T, Koch J, Vygen-Bonnet S, Kulper-Schiek W, Pilic A, Reda S, et al. . Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 may 2021. Euro Surveill (2021) 64(4):383–94. doi: 10.2807/1560-7917.ES.2021.26.28.2100563 - DOI - PMC - PubMed
-
- Hansen CH, Schelde AB, Moustsen-Helm IR, Emborg H-D, Krause TG, Mølbak K, et al. . Vaccine effectiveness against SARS-CoV-2 infection with the omicron or delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. medRxiv (2021). doi: 10.1101/2021.12.20.21267966 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

