The hepatic extramedullary hematopoiesis during experimental murine Schistosomiasis mansoni
- PMID: 36091027
- PMCID: PMC9453041
- DOI: 10.3389/fimmu.2022.955034
The hepatic extramedullary hematopoiesis during experimental murine Schistosomiasis mansoni
Abstract
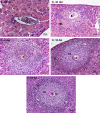
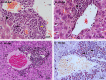
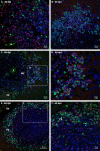
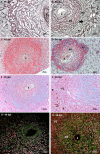
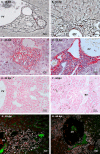
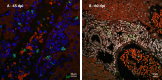
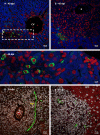
Many years ago, our research group has demonstrated extramedullary hematopoiesis in the peripheral zone of murine hepatic schistosomal granulomas. In the present study, we revisit this phenomenon using new technical and conceptual approaches. Therefore, newborn mice were percutaneously infected by Schistosoma mansoni cercariae and euthanized between 35- and 60-days post infection. Liver samples were submitted to histopathology and immunohistochemical analyses. Cells under mitosis and/or expressing Ki67 demonstrated the proliferation of hematopoietic cells both around the parasite's eggs trapped in the liver and around hepatic vessels. After 50 days post infection, proliferating cells at different levels on differentiation were located preferentially in the peripheral zone of the granulomas, around the vessels and inside the sinusoids. The presence of acidic and sulfated glycoconjugates, reticular fibers and the absence of fibronectin characterized the microenvironment for attraction and maintenance of hematopoiesis. Some neutrophils secreted MMP9 from the earliest points of infection, indicating degradation of the extracellular matrix in regions of histolysis and a possible chemoattraction of hematopoietic stem cells to the liver. Fall-3+ cells and Sca-1+ cells indicated that early hematopoietic progenitors could be mobilized to the liver. Groups of vWF+ megakaryocytes suggest chemoattraction of these cells and/or migration, proliferation, and differentiation of very immature progenitors to this organ. The increase of blood vessels and extramedullary hematopoiesis in this environment, where markers of immature hematopoietic and endothelial cells have been identified, points to the possibility of the presence of progenitors for endothelial and hematopoietic cells in the liver during the infection. There is also the possibility of concomitant migration of more differentiated hematopoietic progenitors, that proliferate and differentiate in the liver, and the occurrence of angiogenesis caused by inflammation or release of ovular antigens that stimulate the activation and proliferation of endothelial cells. Altogether, these data increase knowledge about a murine model that is of interest for investigating the pathology of the schistosomiasis and also the dynamics of hematopoiesis.
Keywords: angiogenesis; extramedullary hematopoiesis; granuloma; hematopoietic environment; schistosomiasis.
Copyright © 2022 Francisco, Terra, Klein, Dias de Oliveira and Pelajo-Machado.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- WHO . Global update on implementation of preventive chemotherapy against neglected tropical diseases in 2018. Weekly Epidemiological Rec No 48 (2021) 96:585–95.
-
- dos Santos CO, Coelho P, Lenzi H. Schistosoma mansoni e esquistossomose uma visaão multidisciplinar. Rio de Janeiro: Editora Fiocruz; (2008) p. 1124 p. Janeiro R de, editor (2008), p. 1124 p.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

