Global research trends on precision cancer medicine-related rashes (2008-2021): A bibliographic study
- PMID: 36091077
- PMCID: PMC9458849
- DOI: 10.3389/fimmu.2022.1002034
Global research trends on precision cancer medicine-related rashes (2008-2021): A bibliographic study
Abstract
Background: Precision cancer medicine-related rashes are a kind of skin and mucous lesions caused by precision therapy. More and more evidences indicated that such events should not be ignored in the course of anti-tumor therapy. Since cancer treatment entered the "Precision Era", there has been a rapid increase in this field. However, there was few bibliometric studies to provide an overall review of this field. This study aims to evaluate the literature output and trends in researches on precision cancer medicine-related rashes from a global perspective.
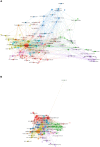
Methods: Collected publications on precision cancer medicine-related rashes from the Web of Science Core Collection database, which were limited to articles and reviews in English. Microsoft Excel, VOS viewer and CiteSpace V were used for quantitative and visual analysis.
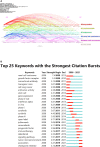
Results: A total of 1,229 papers were identified. From 2008 to 2021, annual publications increased year by year. The United States published the most papers in this field (44.9%) and ranking first in citation frequency (19,854 times) and H-index (69). The University of Texas system ranks first with 98 papers published. Lacouture M.E and Robert C were the principal investigators. Cancers has the largest number of articles published, with 70 articles. In recent years, there have been research hotspots related to immunotherapy, including ipilimumab, immunotherapy, tumor microenvironment, association, checkpoint inhibitor, and cutaneous adverse event.
Conclusion: Precision cancer medicine-related rashes are a hot research topic in oncology. The number of relevant publications will increase dramatically. "Checkpoint inhibitors", "skin adverse events", "associations" and "tumor microenvironment" may become research hotspots in the future.
Keywords: bibliometric analysis; checkpoint inhibitors; precision cancer medicine; rash; targeted therapy.
Copyright © 2022 Zhao, Yu, Chen, Zhao, Sun, Xu, Zhang, Dai, Zhang and Shu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: Nivolumab versus docetaxel in previously treated non-Small-Cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol (2021) 39(7):723–33. doi: 10.1200/jco.20.01605 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

