Full-course resection control strategy in glioma surgery using both intraoperative ultrasound and intraoperative MRI
- PMID: 36091111
- PMCID: PMC9453394
- DOI: 10.3389/fonc.2022.955807
Full-course resection control strategy in glioma surgery using both intraoperative ultrasound and intraoperative MRI
Abstract
Background: Intraoperative ultrasound(iUS) and intraoperative MRI (iMRI) are effective ways to perform resection control during glioma surgery. However, most published studies employed only one modality. Few studies have used both during surgery. How to combine these two techniques reasonably, and what advantages they could have for glioma surgery are still open questions.
Methods: We retrospectively reviewed a series of consecutive patients who underwent initial surgical treatment of supratentorial gliomas in our center. We utilized a full-course resection control strategy to combine iUS and iMRI: IUS for pre-resection assessment and intermediate resection control; iMRI for final resection control. The basic patient characteristics, surgical results, iMRI/iUS findings, and their impacts on surgical procedures were evaluated and reported.
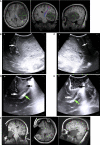
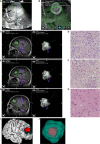
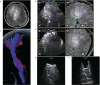
Results: A total of 40 patients were included. The extent of resection was 95.43 ± 10.37%, and the gross total resection rate was 72.5%. The median residual tumor size was 6.39 cm3 (range 1.06-16.23 cm3). 5% (2/40) of patients had permanent neurological deficits after surgery. 17.5% (7/40) of patients received further resection after the first iMRI scan, resulting in four (10%) more patients achieving gross total resection. The number of iMRI scans per patient was 1.18 ± 0.38. The surgical time was 4.5 ± 3.6 hours. The pre-resection iUS scan revealed that an average of 3.8 borders of the tumor were beside sulci in 75% (30/40) patients. Intermediate resection control was utilized in 67.5% (27/40) of patients. In 37.5% (15/40) of patients, the surgical procedures were changed intraoperatively based on the iUS findings. Compared with iMRI, the sensitivity and specificity of iUS for residual tumors were 46% and 96%, respectively.
Conclusion: The full-course resection control strategy by combining iUS and iMRI could be successfully implemented with good surgical results in initial glioma surgeries. This strategy might stabilize resection control quality and provide the surgeon with more intraoperative information to tailor the surgical strategy. Compared with iMRI-assisted glioma surgery, this strategy might improve efficiency by reducing the number of iMRI scans and shortening surgery time.
Keywords: glioma; intraoperative MRI; intraoperative ultrasound; navigation; neurosurgery.
Copyright © 2022 Hou, Li, Li, Yu and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Role of intraoperative ultrasound and MRI to aid grade of resection of pediatric low-grade gliomas: accumulated experience from 4 centers.Childs Nerv Syst. 2024 Oct;40(10):3165-3172. doi: 10.1007/s00381-024-06532-3. Epub 2024 Jul 16. Childs Nerv Syst. 2024. PMID: 39012356
-
The Role of Intraoperative Imaging Modalities in Surgical Resection of Supratentorial Gliomas: A Review of 300 Cases.Oper Neurosurg. 2025 Jun 3. doi: 10.1227/ons.0000000000001628. Online ahead of print. Oper Neurosurg. 2025. PMID: 40459292
-
Challenges and Opportunities of Intraoperative 3D Ultrasound With Neuronavigation in Relation to Intraoperative MRI.Front Oncol. 2021 May 3;11:656519. doi: 10.3389/fonc.2021.656519. eCollection 2021. Front Oncol. 2021. PMID: 34026631 Free PMC article.
-
Intraoperative MRI versus 5-ALA in high-grade glioma resection: a network meta-analysis.J Neurosurg. 2020 Feb 21;134(2):484-498. doi: 10.3171/2019.12.JNS191203. Print 2021 Feb 1. J Neurosurg. 2020. PMID: 32084631 Review.
-
Reliability of intraoperative ultrasound in detecting tumor residual after brain diffuse glioma surgery: a systematic review and meta-analysis.Neurosurg Rev. 2020 Oct;43(5):1221-1233. doi: 10.1007/s10143-019-01160-x. Epub 2019 Aug 14. Neurosurg Rev. 2020. PMID: 31410683
Cited by
-
Application of intraoperative ultrasound in the resection of high-grade gliomas.Front Neurol. 2023 Oct 26;14:1240150. doi: 10.3389/fneur.2023.1240150. eCollection 2023. Front Neurol. 2023. PMID: 37965171 Free PMC article. Review.
-
Progress in the application of ultrasound in glioma surgery.Front Med (Lausanne). 2024 Jun 17;11:1388728. doi: 10.3389/fmed.2024.1388728. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38957299 Free PMC article. Review.
-
Surgical Management and Advances in the Treatment of Glioma.Semin Neurol. 2023 Dec;43(6):810-824. doi: 10.1055/s-0043-1776766. Epub 2023 Nov 14. Semin Neurol. 2023. PMID: 37963582 Free PMC article. Review.
-
Navigated intraoperative ultrasound in pediatric brain tumors.Childs Nerv Syst. 2024 Sep;40(9):2697-2705. doi: 10.1007/s00381-024-06492-8. Epub 2024 Jun 11. Childs Nerv Syst. 2024. PMID: 38862795 Free PMC article.
-
Neuronavigation in glioma resection: current applications, challenges, and clinical outcomes.Front Surg. 2024 Aug 6;11:1430567. doi: 10.3389/fsurg.2024.1430567. eCollection 2024. Front Surg. 2024. PMID: 39165667 Free PMC article.
References
LinkOut - more resources
Full Text Sources

