Potential 18F-RGD PET/CT and DCE-MRI Imaging-Based Biomarkers for Postoperative Survival Prediction Among Patients With Newly Diagnosed Glioblastoma Treated With Bevacizumab and Chemoradiotherapy
- PMID: 36091179
- PMCID: PMC9459034
- DOI: 10.3389/fonc.2022.848266
Potential 18F-RGD PET/CT and DCE-MRI Imaging-Based Biomarkers for Postoperative Survival Prediction Among Patients With Newly Diagnosed Glioblastoma Treated With Bevacizumab and Chemoradiotherapy
Abstract
Purpose: To investigate the ability of potential imaging biomarkers based on 18F-AlF-NOTA-PRGD2 positron emission tomography/computed tomography (18F-RGD PET/CT) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) imaging to predict the response to bevacizumab combined with conventional therapy in postoperative newly diagnosed glioblastoma.
Methods: Twenty patients with newly diagnosed with glioblastoma after surgery were prospectively enrolled to receive bevacizumab plus conventional concurrent radiotherapy and temozolomide (CCRT). 18F-RGD PET/CT and DCE-MRI were performed at baseline, week 3, and week 10 for each patient. Statistical methods included the analysis of variance (ANOVA), Kaplan-Meier method and Cox proportional hazard analysis.
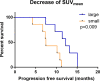
Results: All patients completed CCRT plus bevacizumab therapy without interruption. The median follow-up time was 33.9 months (95% confidence interval [CI], 28.3-39.5 months). The median progression-free survival (PFS) and overall survival (OS) was 9.66 months (95% CI, 6.20-13.12 months) and 15.89 months (95% CI, 13.89-17.78), respectively. Treatment was generally well tolerated, and there were no Treatment emergent adverse events (TEAEs) with a toxicity grade equal to or exceeding 3 or that led to termination of treatment or patient death.Over the treatment interval of bevacizumab therapy from week 3 to week 10, patients with a large decrease of SUVmean was associated with a better PFS with a hazard ratio (HR) of 6.562, 95% CI (1.318-32.667), p=0.022. According to Kaplan-Meier analysis, patients with a decrease in the SUVmean of more than 0.115 on 18F-RGD PET/CT had a longer PFS than those with a decrease in the SUVmean of 0.115 or less (12.25 months vs.7.46 months, p=0.009). For OS, only a small decrease of Ktrans was also found to have certain prognostic value (HR=0.986, 95% CI (0.975-0.998), p=0.023). Patients with a decrease in Ktrans larger than 37.03 (min-1) on DCE-MRI had worse OS than those with a decrease in Ktrans of 37.03 (min-1) or less (15.93 months vs. 26.42 months, p=0.044).
Conclusion: 18F-RGD PET/CT and DCE-MRI may be valuable in evaluating the response of glioblastoma to treatment with the combination of bevacizumab and CCRT, with a greater decrease in SUVmean predicting better PFS as well as a small decrease in Ktrans predicting improved OS.
Keywords: 18F-RGD PET/CT; DCE-MRI; Glioblastoma; OS; PFS; bevacizumab; concurrent radiotherapy and temozolomide.
Copyright © 2022 Li, Liu, Zhang, Tao, Zhao, Chen, Fu, Li, Xu, Liu, Yu and Yuan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Takano S, Yoshii Y, Kondo S, Suzuki H, Maruno T, Shirai S, et al. Concentration of Vascular Endothelial Growth Factor in the Serum and Tumor Tissue of Brain Tumor Patients. Cancer Res (1996) 56(9):2185–90. - PubMed
-
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-Dependent Vascular Regression and Permeability Changes in Established Human Tumor Xenografts Induced by an Anti-Vascular Endothelial Growth Factor/Vascular Permeability Factor Antibody. Proc Natl Acad Sci USA (1996) 93(25):14765–70. doi: 10.1073/pnas.93.25.14765 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

