Fabrication of graphene-based porous materials: traditional and emerging approaches
- PMID: 36091205
- PMCID: PMC9365090
- DOI: 10.1039/d2sc01786e
Fabrication of graphene-based porous materials: traditional and emerging approaches
Abstract
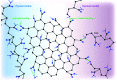
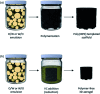
The anisotropic nature of 'graphenic' nanosheets enables them to form stable three-dimensional porous materials. The use of these porous structures has been explored in several applications including electronics and batteries, environmental remediation, energy storage, sensors, catalysis, tissue engineering, and many more. As method of fabrication greatly influences the final pore architecture, and chemical and mechanical characteristics and performance of these porous materials, it is essential to identify and address the correlation between property and function. In this review, we report detailed analyses of the different methods of fabricating porous graphene-based structures - with a focus on graphene oxide as the base material - and relate these with the resultant morphologies, mechanical responses, and common applications of use. We discuss the feasibility of the synthesis approaches and relate the GO concentrations used in each methodology against their corresponding pore sizes to identify the areas not explored to date.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
N. T. holds a patent on the use of graphene sponges for water treatment. All other authors have no conflicts to declare.
Figures






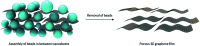



References
-
- Li Y. Cui W. Liu L. Zong R. Yao W. Liang Y. Zhu Y. Removal of Cr(VI) by 3D TiO 2 -graphene hydrogel via adsorption enriched with photocatalytic reduction. Appl. Catal., B. 2016;199:412. doi: 10.1016/j.apcatb.2016.06.053. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

