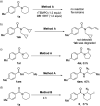
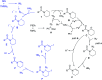
Selective desaturation of amides: a direct approach to enamides
- PMID: 36091215
- PMCID: PMC9365091
- DOI: 10.1039/d2sc02210a
Selective desaturation of amides: a direct approach to enamides
Abstract
C(sp3)-H bond desaturation has been an attractive strategy in organic synthesis. Enamides are important structural fragments in pharmaceuticals and versatile synthons in organic synthesis. However, the dehydrogenation of amides usually occurs on the acyl side benefitting from enolate chemistry like the desaturation of ketones and esters. Herein, we demonstrate an Fe-assisted regioselective oxidative desaturation of amides, which provides an efficient approach to enamides and β-halogenated enamides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
A facile synthesis of α,β-unsaturated imines via palladium-catalyzed dehydrogenation.Nat Commun. 2024 May 21;15(1):4329. doi: 10.1038/s41467-024-48737-9. Nat Commun. 2024. PMID: 38773128 Free PMC article.
-
Catalytic dehydrogenative synthesis of α,β-unsaturated secondary amides without external oxidants.Chem Sci. 2024 Aug 23;15(37):15385-90. doi: 10.1039/d4sc04419c. Online ahead of print. Chem Sci. 2024. PMID: 39246373 Free PMC article.
-
Exploring tertiary enamides as versatile synthons in organic synthesis.Chem Commun (Camb). 2015 Apr 11;51(28):6039-49. doi: 10.1039/c4cc10327k. Chem Commun (Camb). 2015. PMID: 25673494 Review.
-
Direct metal-catalyzed regioselective functionalization of enamides.Chemistry. 2014 Jun 16;20(25):7548-64. doi: 10.1002/chem.201402070. Epub 2014 May 26. Chemistry. 2014. PMID: 24862089 Review.
-
Tertiary Enamides: Versatile and Available Substrates in Synthetic Chemistry.Curr Org Synth. 2019;16(1):70-97. doi: 10.2174/1570179415666181107122814. Curr Org Synth. 2019. PMID: 31965923
Cited by
-
α,β-Desaturation and Formal β-C(sp3)-H Fluorination of N-Substituted Amines: A Late-Stage Functionalization Strategy Enabled by Electrochemistry.J Am Chem Soc. 2024 Aug 21;146(33):22982-22992. doi: 10.1021/jacs.4c02548. Epub 2024 Aug 12. J Am Chem Soc. 2024. PMID: 39132893 Free PMC article.
-
Recent Progress in Synthetic Applications of Hypervalent Iodine(III) Reagents.Chem Rev. 2024 Oct 9;124(19):11108-11186. doi: 10.1021/acs.chemrev.4c00303. Epub 2024 Sep 13. Chem Rev. 2024. PMID: 39269928 Free PMC article. Review.
-
A facile synthesis of α,β-unsaturated imines via palladium-catalyzed dehydrogenation.Nat Commun. 2024 May 21;15(1):4329. doi: 10.1038/s41467-024-48737-9. Nat Commun. 2024. PMID: 38773128 Free PMC article.
References
-
- Carbery D. R. Org. Biomol. Chem. 2008;6:3455–3460. doi: 10.1039/B809319A. - DOI - PubMed
- Gopalaiah K. Kagan H. B. Chem. Rev. 2011;111:4599–4657. doi: 10.1021/cr100031f. - DOI - PubMed
- Masson G. Bernadat G. Synlett. 2014;25:2842–2867. doi: 10.1055/s-0034-1379166. - DOI
- Masson G. Courant T. Dagousset G. Synthesis. 2015;47:1799–1856. doi: 10.1055/s-0034-1378706. - DOI
- Wang M. X. Chem. Commun. 2015;51:6039–6049. doi: 10.1039/C4CC10327K. - DOI - PubMed
-
- Linstead R. P. Braude E. A. Mitchell P. W. D. Wooldridge K. R. H. Jackman L. M. Nature. 1952;169:100–103. doi: 10.1038/169100a0. - DOI
- Mamedov E. A. Cortés Corberán V. Appl. Catal., A. 1995;127:1–40. doi: 10.1016/0926-860X(95)00056-9. - DOI
- Kung H. H. Kung M. C. Appl. Catal., A. 1997;157:105–116. doi: 10.1016/S0926-860X(97)00028-8. - DOI
- Madeira L. M. Portela M. F. Catal. Rev. 2002;44:247–286. doi: 10.1081/CR-120001461. - DOI
- Zhang X. Fried A. Knapp S. Goldman A. S. Chem. Commun. 2003:2060–2061. doi: 10.1039/B304357F. - DOI - PubMed
- Wang S. Zhu Z. H. Energy Fuels. 2004;18:1126–1139. doi: 10.1021/ef0340716. - DOI
- Ansari M. B. Park S.-E. Energy Environ. Sci. 2012;5:9419. doi: 10.1039/C2EE22409G. - DOI
- Schümperli M. T. Hammond C. Hermans I. ACS Catal. 2012;2:1108–1117. doi: 10.1021/cs300212q. - DOI
- Voica A.-F. Mendoza A. Gutekunst W. R. Fraga J. O. Baran P. S. Nat. Chem. 2012;4:629–635. doi: 10.1038/nchem.1385. - DOI - PMC - PubMed
- Wertz S. Studer A. Green Chem. 2013;15:3116. doi: 10.1039/C3GC41459K. - DOI
- Chen B. Wang L. Gao S. ACS Catal. 2015;5:5851–5876. doi: 10.1021/acscatal.5b01479. - DOI
- Sheldon R. A. Catal. Today. 2015;247:4–13. doi: 10.1016/j.cattod.2014.08.024. - DOI
- Wendlandt A. E. Stahl S. S. Angew. Chem., Int. Ed. 2015;54:14638–14658. doi: 10.1002/anie.201505017. - DOI - PMC - PubMed
- Zhou M. J. Zhu S. F. Zhou Q. L. Chem. Commun. 2017;53:8770–8773. doi: 10.1039/C7CC04761D. - DOI - PubMed
-
- Göttker-Schnetmann I. White P. Brookhart M. J. Am. Chem. Soc. 2004;126:1804–1811. doi: 10.1021/ja0385235. - DOI - PubMed
- Bolig A. D. Brookhart M. J. Am. Chem. Soc. 2007;129:14544–14545. doi: 10.1021/ja075694r. - DOI - PMC - PubMed
- Giri R. Maugel N. Foxman B. M. Yu J.-Q. Organometallics. 2008;27:1667–1670. doi: 10.1021/om8000444. - DOI
- Dobereiner G. E. Crabtree R. H. Chem. Rev. 2010;110:681–703. doi: 10.1021/cr900202j. - DOI - PubMed
- Johnson T. C. Morris D. J. Wills M. Chem. Soc. Rev. 2010;39:81–88. doi: 10.1039/B904495G. - DOI - PubMed
- Choi J. MacArthur A. H. Brookhart M. Goldman A. S. Chem. Rev. 2011;111:1761–1779. doi: 10.1021/cr1003503. - DOI - PubMed
- Gunanathan C. Milstein D. Science. 2013;341:1229712. doi: 10.1126/science.1229712. - DOI - PubMed
- Bheeter C. B. Jin R. Bera J. K. Dixneuf P. H. Doucet H. Adv. Synth. Catal. 2014;356:119–124. doi: 10.1002/adsc.201300795. - DOI
- Yao W. Zhang Y. Jia X. Huang Z. Angew. Chem., Int. Ed. 2014;53:1390–1394. doi: 10.1002/anie.201306559. - DOI - PubMed
- Werkmeister S. Neumann J. Junge K. Beller M. Chemistry. 2015;21:12226–12250. doi: 10.1002/chem.201500937. - DOI - PubMed
- Kumar A. Bhatti T. M. Goldman A. S. Chem. Rev. 2017;117:12357–12384. doi: 10.1021/acs.chemrev.7b00247. - DOI - PubMed
- Huang L. Bismuto A. Rath S. A. Trapp N. Morandi B. Angew. Chem., Int. Ed. 2021;60:7290–7296. doi: 10.1002/anie.202015837. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources