Gypenosides ameliorate high-fat diet-induced non-alcoholic steatohepatitis via farnesoid X receptor activation
- PMID: 36091227
- PMCID: PMC9449333
- DOI: 10.3389/fnut.2022.914079
Gypenosides ameliorate high-fat diet-induced non-alcoholic steatohepatitis via farnesoid X receptor activation
Abstract
Background: Gypenosides (Gyps), the major botanical component of Gynostemma pentaphyllum, was found to up-regulate the farnesoid X receptor (FXR) in a mouse model of non-alcoholic steatohepatitis (NASH). However, the exact role of FXR and underlying mechanisms in Gyps-mediated effects on NASH remain to be elucidated.
Purpose: This study investigated whether Gyps attenuates NASH through directly activating FXR in high-fat diet (HFD)-induced NASH, and delineated the molecular pathways involved.
Study design: A mouse model of HFD-induced NSAH was used to examine effects of Gyps on NASH with obeticholic acid (OCA) as a positive control, and the role of FXR in its mechanism of action was investigated in wild-type (WT) and FXR knockout (KO) mice.
Methods: WT or FXR KO mice were randomly assigned into four groups: normal diet (ND) group as negative control, HFD group, HFD + Gyps group, or HFD + OCA group.
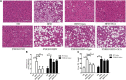
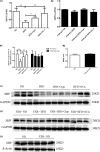
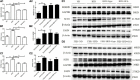
Results: Treatment with Gyps and OCA significantly improved liver histopathological abnormalities in HFD-induced NASH, reduced the non-alcoholic fatty liver disease (NAFLD) activity score (NAS), and lowered hepatic triglyceride (TG) content compared with the HFD group. In agreement with these liver tissue changes, biochemical tests of blood samples revealed that alanine aminotransferase (ALT), aspartate aminotransferase (AST), TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (FBG), and fasting insulin (FINS) levels were significantly lower in the HFD + Gyps vs. HFD group. Furthermore, Gyps and OCA treatment significantly up-regulated hepatic FXR, small heterodimer partner (SHP), carnitine palmitoyltransferase 1A (CPT1A), and lipoprotein lipase (LPL) expression, and significantly down-regulated sterol-regulatory element binding protein 1 (SREBP1), fatty acid synthetase (FASN), and stearoyl-CoA desaturase 1 (SCD1) protein levels compared with the HFD group in WT mice but not in FXR KO mice. Notably, Gyps- and OCA-mediated pharmacological effects were significantly abrogated by depletion of the FXR gene in FXR KO mice.
Conclusion: Gyps ameliorated HFD-induced NASH through the direct activation of FXR and FXR-dependent signaling pathways.
Keywords: farnesoid X receptor; gypenosides; high-fat diet; mice; non-alcoholic steatohepatitis.
Copyright © 2022 Li, Xi, Liu and Xin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

