Analysis of the initial dose and reduction rate of corticosteroid for ulcerative colitis in clinical practice
- PMID: 36091323
- PMCID: PMC9446402
- DOI: 10.1002/jgh3.12796
Analysis of the initial dose and reduction rate of corticosteroid for ulcerative colitis in clinical practice
Abstract
Background and aim: Trends in steroid use and the effects of the initial dose, duration of use, and tapering schedule on clinical efficacy were assessed in Japanese patients with ulcerative colitis (UC) undergoing steroid treatment.
Methods: We enrolled 191 cases with UC who underwent steroid treatment between 2006 and 2020. We assessed the difference in clinical remission rates in cases with different initial doses of steroid. Clinical factors for clinical remission at week 4 and discontinuation of corticosteroid within 12 weeks were also assessed.
Results: Clinical remission and response at week 4 were obtained in 107 (56.0%) and 58 cases (30.4%), respectively. In hospitalized patients, male sex (odds ratio [OR], 0.373; 95% confidence interval [CI], 0.146-0.956) and younger age (OR, 0.974; 95% CI, 0.951-0.998) were associated with clinical remission at week 4. Partial Mayo score (OR, 0.643; 95% CI, 0.451-0.918) and initial steroid dose of ≥30 mg (OR, 3.278; 95% CI, 1.274-8.435) were associated with clinical remission at week 4 in outpatients. Clinical remission at week 4 (OR, 0.300; (95% CI, 0.126-0.718)) and the steroid dose reduction rate at week 4 (OR, 0.092; 95% CI, 0.036-0.234) were associated with treatment discontinuation within 12 weeks. The proportion of patients in whom corticosteroids were discontinued at week 12 was significantly higher (P = 0.006) in 2016-2020 (28/52; 53.8%) than in 2006-2010 (15/54; 27.8%).
Conclusion: The steroid reduction rate at week 4 may be critical for discontinuation within 12 weeks. Withdrawal of corticosteroids has been becoming more appropriate in the last 5 years than before.
Keywords: corticosteroid; initial dose of corticosteroid; reduction rate of corticosteroid; ulcerative colitis.
© 2022 The Authors. JGH Open published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Figures
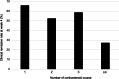
 ), CR (+); (
), CR (+); ( ), CR (−)
), CR (−)
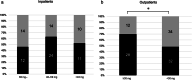
 ), CR (+); (
), CR (+); ( ), CR (−). (b): (
), CR (−). (b): ( ), ≤20 mg; (
), ≤20 mg; ( ), 21–40 mg; (
), 21–40 mg; ( ), 41 mg. (c): (
), 41 mg. (c): ( ), 0–49%; (
), 0–49%; ( ), 50–100%. (d): (
), 50–100%. (d): ( ), 0–99%; (
), 0–99%; ( ), discontinuation.
), discontinuation.References
-
- Naganuma M, Mizuno S, Nanki K, Sugimoto S, Kanai T. Recent trends and future directions for the medical treatment of ulcerative colitis. Clin. J. Gastroenterol. 2016; 9: 329–36. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005; 353: 2462–76. - PubMed
-
- Sandborn WJ, van Assche G, Reinisch W et al. Adalimumab induces and maintains clinical remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2012; 142: 257–65. - PubMed
-
- Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2014; 146: 85–95. - PubMed
-
- Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab maintains clinical response in patients with moderate‐to‐severe ulcerative colitis. Gastroenterology. 2014; 146: 96–109.e1. - PubMed
LinkOut - more resources
Full Text Sources

