The impact of COVID-19 on populations living at high altitude: Role of hypoxia-inducible factors (HIFs) signaling pathway in SARS-CoV-2 infection and replication
- PMID: 36091390
- PMCID: PMC9454615
- DOI: 10.3389/fphys.2022.960308
The impact of COVID-19 on populations living at high altitude: Role of hypoxia-inducible factors (HIFs) signaling pathway in SARS-CoV-2 infection and replication
Abstract
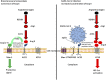
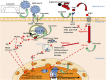
Cases of coronavirus disease 2019 (COVID-19) have been reported worldwide. However, one epidemiological report has claimed a lower incidence of the disease in people living at high altitude (>2,500 m), proposing the hypothesis that adaptation to hypoxia may prove to be advantageous with respect to SARS-CoV-2 infection. This publication was initially greeted with skepticism, because social, genetic, or environmental parametric variables could underlie a difference in susceptibility to the virus for people living in chronic hypobaric hypoxia atmospheres. Moreover, in some patients positive for SARS-CoV-2, early post-infection 'happy hypoxia" requires immediate ventilation, since it is associated with poor clinical outcome. If, however, we accept to consider the hypothesis according to which the adaptation to hypoxia may prove to be advantageous with respect to SARS-CoV-2 infection, identification of the molecular rational behind it is needed. Among several possibilities, HIF-1 regulation appears to be a molecular hub from which different signaling pathways linking hypoxia and COVID-19 are controlled. Interestingly, HIF-1α was reported to inhibit the infection of lung cells by SARS-CoV-2 by reducing ACE2 viral receptor expression. Moreover, an association of the rs11549465 variant of HIF-1α with COVID-19 susceptibility was recently discovered. Here, we review the evidence for a link between HIF-1α, ACE2 and AT1R expression, and the incidence/severity of COVID-19. We highlight the central role played by the HIF-1α signaling pathway in the pathophysiology of COVID-19.
Keywords: COVID-19; Nrf2; TRPA1; curcumin; hypoxia; hypoxia-inducible factors.
Copyright © 2022 Devaux and Raoult.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Unraveling the Underlying Molecular Mechanism of 'Silent Hypoxia' in COVID-19 Patients Suggests a Central Role for Angiotensin II Modulation of the AT1R-Hypoxia-Inducible Factor Signaling Pathway.J Clin Med. 2023 Mar 22;12(6):2445. doi: 10.3390/jcm12062445. J Clin Med. 2023. PMID: 36983445 Free PMC article. Review.
-
HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19.Signal Transduct Target Ther. 2021 Aug 18;6(1):308. doi: 10.1038/s41392-021-00726-w. Signal Transduct Target Ther. 2021. PMID: 34408131 Free PMC article.
-
Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets.Acta Pharmacol Sin. 2020 Dec;41(12):1539-1546. doi: 10.1038/s41401-020-00554-8. Epub 2020 Oct 27. Acta Pharmacol Sin. 2020. PMID: 33110240 Free PMC article. Review.
-
Chemotherapy induces ACE2 expression in breast cancer via the ROS-AKT-HIF-1α signaling pathway: a potential prognostic marker for breast cancer patients receiving chemotherapy.J Transl Med. 2022 Nov 5;20(1):509. doi: 10.1186/s12967-022-03716-w. J Transl Med. 2022. PMID: 36335375 Free PMC article.
-
Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis.Cell Metab. 2020 Sep 1;32(3):437-446.e5. doi: 10.1016/j.cmet.2020.07.007. Epub 2020 Jul 17. Cell Metab. 2020. PMID: 32697943 Free PMC article.
Cited by
-
Oxidative Stress Enhances Rubella Virus Infection in Immortalized Human First-Trimester Trophoblasts.Int J Mol Sci. 2025 Jan 25;26(3):1041. doi: 10.3390/ijms26031041. Int J Mol Sci. 2025. PMID: 39940811 Free PMC article.
-
Effect of apigetrin in pseudo-SARS-CoV-2-induced inflammatory and pulmonary fibrosis in vitro model.Sci Rep. 2024 Jun 24;14(1):14545. doi: 10.1038/s41598-024-65447-w. Sci Rep. 2024. PMID: 38914619 Free PMC article.
-
An Ecological Study Relating the SARS-CoV-2 Epidemiology with Health-Related, Socio-Demographic, and Geographical Characteristics in South Tyrol (Italy).Int J Environ Res Public Health. 2024 Nov 30;21(12):1604. doi: 10.3390/ijerph21121604. Int J Environ Res Public Health. 2024. PMID: 39767445 Free PMC article.
-
Unraveling the Underlying Molecular Mechanism of 'Silent Hypoxia' in COVID-19 Patients Suggests a Central Role for Angiotensin II Modulation of the AT1R-Hypoxia-Inducible Factor Signaling Pathway.J Clin Med. 2023 Mar 22;12(6):2445. doi: 10.3390/jcm12062445. J Clin Med. 2023. PMID: 36983445 Free PMC article. Review.
-
Expression and diagnostic values of ferroptosis-related genes in coronavirus-associated viral sepsis.Front Med (Lausanne). 2025 Apr 30;12:1496834. doi: 10.3389/fmed.2025.1496834. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40370730 Free PMC article.
References
-
- Ahmadi R., Salari S., Sharifi M. D., Reihani H., Rostamiani M. B., Behmadi M., et al. (2021). Oral nano‐curcumin formulation efficacy in the management of mild to moderate outpatient COVID‐ 19: A randomized triple‐blind placebo‐controlled clinical trial. Food Sci. Nutr. 9 (8), 4068–4075. 10.1002/fsn3.2226 - DOI - PMC - PubMed
-
- Arendse L. B., Danser A. H. J., Poglitsch M., Touyz R. M., Burnett J. C., Llorens-Cortes C., et al. (2019). Novel therapeutic approaches targeting the Renin-Angiotensin System and associated peptides in hypertension and heart failure. Pharmacol. Rev. 71, 539–570. 10.1124/pr.118.017129 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

