A sensitive mitochondrial thermometry 2.0 and the availability of thermogenic capacity of brown adipocyte
- PMID: 36091398
- PMCID: PMC9449420
- DOI: 10.3389/fphys.2022.977431
A sensitive mitochondrial thermometry 2.0 and the availability of thermogenic capacity of brown adipocyte
Abstract
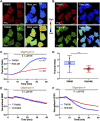
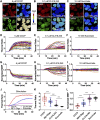
The temperature of a living cell is a crucial parameter for cellular events, such as cell division, gene expressions, enzyme activities and metabolism. We previously developed a quantifiable mitochondrial thermometry 1.0 based on rhodamine B methyl ester (RhB-ME) and rhodamine 800 (Rh800), and the theory for mitochondrial thermogenesis. Given that the synthesized RhB-ME is not readily available, thus, a convenient mitochondrial thermometry 2.0 based on tetra-methyl rhodamine methyl ester (TMRM) and Rh800 for the thermogenic study of brown adipocyte was further evolved. The fluorescence of TMRM is more sensitive (∼1.4 times) to temperature than that of RhB-ME, then the TMRM-based mito-thermometry 2.0 was validated and used for the qualitatively dynamic profiles for mitochondrial thermogenic responses and mitochondrial membrane potential in living cells simultaneously. Furthermore, our results demonstrated that the heterogenous thermogenesis evoked by β3 adrenoceptor agonist only used overall up to ∼46% of the thermogenic capacity evoked by CCCP stimulation. On the other hand, the results demonstrated that the maximum thermogenesis evoked by NE and oligomycin A used up to ∼79% of the thermogenic capacity, which suggested the maximum thermogenic capacity under physiological conditions by inhibiting the proton-ATPase function of the mitochondrial complex V, such as under the cold activation of sympathetic nerve and the co-release of sympathetic transmitters.
Keywords: Rh800; TMRM; brown adipocytes; mitochondrial thermometry 2.0; thermogenesis.
Copyright © 2022 Meng, Wang, Xie, Yang, Liu, Liu, Li, Luan and Kang.
Conflict of interest statement
CFL is employed by the Company RevoImmune Therapeutics Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Barbosa M. A., Guerra-Sá R., De Castro U. G. M., de Lima W. G., dos Santos R. A. S., Campagnole-Santos M. J., et al. (2018). Physical training improves thermogenesis and insulin pathway, and induces remodeling in white and Brown adipose tissues. J. Physiol. Biochem. 74, 441–454. 10.1007/s13105-018-0637-x PubMed Abstract | 10.1007/s13105-018-0637-x | Google Scholar - DOI - DOI - PubMed
-
- Blüher M. (2019). Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. 10.1038/s41574-019-0176-8 PubMed Abstract | 10.1038/s41574-019-0176-8 | Google Scholar - DOI - DOI - PubMed
-
- Chazotte B. (2011). Labeling mitochondria with TMRM or TMRE. Cold Spring Harb. Protoc. 2011, 895–897. 10.1101/pdb.prot5641 PubMed Abstract | 10.1101/pdb.prot5641 | Google Scholar - DOI - DOI - PubMed
-
- Chouchani E. T., Kazak L., Jedrychowski M. P., Lu G. Z., Erickson B. K., Szpyt J., et al. (2016). Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116. 10.1038/nature17399 PubMed Abstract | 10.1038/nature17399 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Chrétien D., Bénit P., Leroy C., El-Khoury R., Park S., Lee J. Y., et al. (2020). Pitfalls in monitoring mitochondrial temperature using charged thermosensitive fluorophores. Chemosensors 8, 124. 10.3390/chemosensors8040124 10.3390/chemosensors8040124 | Google Scholar - DOI - DOI
LinkOut - more resources
Full Text Sources

