Immunomodulatory effects of new phytotherapy on human macrophages and TLR4- and TLR7/8-mediated viral-like inflammation in mice
- PMID: 36091684
- PMCID: PMC9450044
- DOI: 10.3389/fmed.2022.952977
Immunomodulatory effects of new phytotherapy on human macrophages and TLR4- and TLR7/8-mediated viral-like inflammation in mice
Abstract
Background: While all efforts have been undertaken to propagate the vaccination and develop remedies against SARS-CoV-2, no satisfactory management of this infection is available yet. Moreover, poor availability of any preventive and treatment measures of SARS-CoV-2 in economically disadvantageous communities aggravates the course of the pandemic. Here, we studied a new immunomodulatory phytotherapy (IP), an extract of blackberry, chamomile, garlic, cloves, and elderberry as a potential low-cost solution for these problems given the reported efficacy of herbal medicine during the previous SARS virus outbreak.
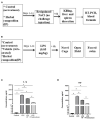
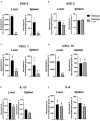
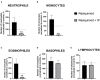
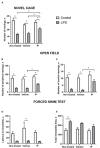
Methods: The key feature of SARS-CoV-2 infection, excessive inflammation, was studied in in vitro and in vivo assays under the application of the IP. First, changes in tumor-necrosis factor (TNF) and lnteurleukin-1 beta (IL-1β) concentrations were measured in a culture of human macrophages following the lipopolysaccharide (LPS) challenge and treatment with IP or prednisolone. Second, chronically IP-pre-treated CD-1 mice received an agonist of Toll-like receptors (TLR)-7/8 resiquimod and were examined for lung and spleen expression of pro-inflammatory cytokines and blood formula. Finally, chronically IP-pre-treated mice challenged with LPS injection were studied for "sickness" behavior. Additionally, the IP was analyzed using high-potency-liquid chromatography (HPLC)-high-resolution-mass-spectrometry (HRMS).
Results: LPS-induced in vitro release of TNF and IL-1β was reduced by both treatments. The IP-treated mice displayed blunted over-expression of SAA-2, ACE-2, CXCL1, and CXCL10 and decreased changes in blood formula in response to an injection with resiquimod. The IP-treated mice injected with LPS showed normalized locomotion, anxiety, and exploration behaviors but not abnormal forced swimming. Isoquercitrin, choline, leucine, chlorogenic acid, and other constituents were identified by HPLC-HRMS and likely underlie the IP immunomodulatory effects.
Conclusions: Herbal IP-therapy decreases inflammation and, partly, "sickness behavior," suggesting its potency to combat SARS-CoV-2 infection first of all via its preventive effects.
Keywords: SARS-CoV-2; inflammation; mice; pro-inflammatory cytokines; toll-like receptors.
Copyright © 2022 Schapovalova, Gorlova, de Munter, Sheveleva, Eropkin, Gorbunov, Sicker, Umriukhin, Lyubchyk, Lesch, Strekalova and Schroeter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Fongnzossie Fedoung E, Biwole AB, Nyangono Biyegue CF, Ngansop Tounkam M, Akono Ntonga P, Nguiamba VP, et al. A review of cameroonian medicinal plants with potentials for the management of the COVID-19 pandemic. Adv Tradit Med. (2021) 26:1–26. 10.1007/s13596-021-00567-6 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

