Combined hyperforin and lanicemine treatment instead of ketamine or imipramine restores behavioral deficits induced by chronic restraint stress and dietary zinc restriction in mice
- PMID: 36091748
- PMCID: PMC9448861
- DOI: 10.3389/fphar.2022.933364
Combined hyperforin and lanicemine treatment instead of ketamine or imipramine restores behavioral deficits induced by chronic restraint stress and dietary zinc restriction in mice
Abstract
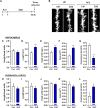
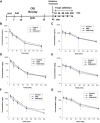
Clinical and preclinical studies show evidence that chronic stress or nutritional deficits in dietary zinc (Zn) intake may be risk factors for developing major depressive disorder (MDD). Furthermore, there may be possible links between low serum Zn levels and development of treatment-resistant depression. In the present work, we combined chronic restraint stress (CRS) and a low-zinc diet (ZnD) in mice and carried out a set of behavioral and biochemical studies. The mice were treated with four different antidepressant compounds, namely, ketamine, Ro 25-6981 (Ro), hyperforin and lanicemine (Hyp + Lan), and imipramine (IMI). We show that CRS or ZnD alone or a combination of CRS and ZnD (CRS + ZnD) induces anhedonia observed in the sucrose preference test (SPT). The behavioral effects of CRS were restored by ketamine or IMI. However, only Hyp + Lan restored the deficits in behavioral phenotype in mice subjected to CRS + ZnD. We also showed that the antidepressant-like effects observed in Hyp + Lan-treated CRS + ZnD mice were associated with changes in the morphology of the dendritic spines (restored physiological level) in the hippocampus (Hp). Finally, we studied the metabolism of ketamine and its brain absorption in CRS and CRS + ZnD mice. Our results suggest that CRS + ZnD does not alter the metabolism of ketamine to (2R,6R;2S,6S)-HNK; however, CRS + ZnD can induce altered bioavailability and distribution of ketamine in the Hp and frontal cortex (FC) in CRS + ZnD animals compared to the control and CRS groups.
Keywords: chronic stress; hyperforin and lanicemine; ketamine; refractory depression; zinc deficiency.
Copyright © 2022 Pochwat, Misztak, Masternak, Bączyńska, Bijata, Roszkowska, Bijata, Włodarczyk, Szafarz, Wyska, Muszyńska, Krakowska, Opoka, Nowak and Szewczyk.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bijata M., Baczynska E., Muller F. E., Bijata K., Masternak J., Krzystyniak A., et al. (2022). Activation of the 5-HT7 receptor and MMP-9 signaling module in the hippocampal CA1 region is necessary for the development of depressive-like behavior. Cell Rep. 38, 110532. 10.1016/j.celrep.2022.110532 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

