Chinese herbal medicine combined with oxaliplatin-based chemotherapy for advanced gastric cancer: A systematic review and meta-analysis of contributions of specific medicinal materials to tumor response
- PMID: 36091754
- PMCID: PMC9453215
- DOI: 10.3389/fphar.2022.977708
Chinese herbal medicine combined with oxaliplatin-based chemotherapy for advanced gastric cancer: A systematic review and meta-analysis of contributions of specific medicinal materials to tumor response
Abstract
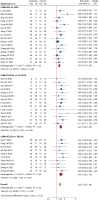
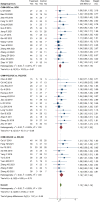
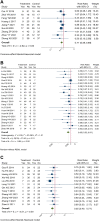
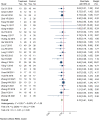
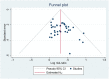
Introduction: The incidence and mortality of gastric cancer ranks among the highest, and the 5-year survival rate of advanced gastric cancer (AGC) is less than 10%. Currently, chemotherapy is the main treatment for AGC, and oxaliplatin is an important part of the commonly used chemotherapy regimen for AGC. A large number of RCTs have shown that Chinese herbal medicine (CHM) combined with oxaliplatin-based chemotherapy can improve objective response rate (ORR) and disease control rate (DCR), reduce the toxic and side effects of chemotherapy. There is currently a lack of systematic evaluation of the evidence to account for the efficacy and safety of CHM combined with oxaliplatin-based chemotherapy in AGC. Therefore, we carried out this study and conducted the sensitivity analysis on the herbal composition to explore the potential anti-tumor efficacy. Methods: Databases of PubMed, EMBASE, CENTRAL, Web of Science, the Chinese Biomedical Literature Database, the China National Knowledge Infrastructure, the Wanfang database, and the Chinese Scientific Journals Database were searched from their inception to April 2022. RCTs evaluating the efficacy of CHM combined with oxaliplatin-based chemotherapy on AGC were included. Stata 16 was used for data synthesis, RoB 2 for quality evaluation of included RCTs, and GRADE for quality of synthesized evidence. Additional sensitivity analysis was performed to explore the potential anti-tumor effects of single herbs and combination of herbs. Results: Forty trials involving 3,029 participants were included. Most included RCTs were assessed as "Some concerns" of risk of bias. Meta-analyses showed that compare to oxaliplatin-based chemotherapy alone, that CHM combined with oxaliplatin-based chemotherapy could increase the objective response rate (ORR) by 35% [risk ratio (RR) = 1.35, 95% confidence intervals (CI) (1.25, 1.45)], and disease control rate (DCR) by 12% [RR = 1.12, 95% CI (1.08, 1.16)]. Subgroup analysis showed that compare to SOX, FOLFOX, and XELOX regimens alone, CHM plus SOX, CHM plus FOLFOX, and CHM plus XELOX could significantly increase the ORR and DCR. Sensitivity analysis identified seven herbs of Astragalus, Liquorice, Poria, Largehead Atractylodes, Chinese Angelica, Codonopsis, and Tangerine Peel with potentials to improve tumor response of oxaliplatin-based chemotherapy in AGC. Conclusion: Synthesized evidence showed moderate certainty that CHM plus oxaliplatin-based chemotherapy may promote improvement in tumor response in AGC. CHM treatment is safe for AGC. Due to the poor quality of included RCTs and small samplesizes, the quality of synthesized evidence was not high. Specific combinations of herbs appeared to produce higher contributions to ORR than the herb individually. Each of this seven above mentioned herbs has been shown in experimental studies to potentially contribute to the improvement of tumor response. To support this conclusion, these seven herbs are worthy of further clinical research. Systematic Review Registration: [http://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=262595], identifier [CRD42022262595].
Keywords: Chinese herbal medicine; advanced gastric cancer; efficacy; meta-analysis; oxaliplatin; synergistic action; tumor response.
Copyright © 2022 Tan, Wang, Xu, Zhang, Zhu, Ge, Lu, Gao and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer SC declared a shared parent affifiliation with the authors BX, GZ, YG, and TL at the time of review.
Figures



Similar articles
-
Efficacy and Safety of Compound Kushen Injection for Advanced Colorectal Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials with Trial Sequential Analysis.Integr Cancer Ther. 2024 Jan-Dec;23:15347354241258458. doi: 10.1177/15347354241258458. Integr Cancer Ther. 2024. PMID: 38853681 Free PMC article.
-
Efficacy and safety of Atractylodes macrocephala-containing traditional Chinese medicine combined with neoadjuvant chemotherapy in the treatment of advanced gastric cancer: a systematic evaluation and meta-analysis.Front Oncol. 2024 Oct 16;14:1431381. doi: 10.3389/fonc.2024.1431381. eCollection 2024. Front Oncol. 2024. PMID: 39479020 Free PMC article.
-
Efficacy and Safety of Astragalus-Containing Traditional Chinese Medicine Combined With Platinum-Based Chemotherapy in Advanced Gastric Cancer: A Systematic Review and Meta-Analysis.Front Oncol. 2021 Aug 4;11:632168. doi: 10.3389/fonc.2021.632168. eCollection 2021. Front Oncol. 2021. PMID: 34422628 Free PMC article.
-
Efficacy and safety of Chinese herbal medicine in post-stroke epilepsy: a systematic review and meta-analysis.Front Pharmacol. 2023 Nov 21;14:1286093. doi: 10.3389/fphar.2023.1286093. eCollection 2023. Front Pharmacol. 2023. PMID: 38074155 Free PMC article.
-
Efficacy and Safety of Xiao Ai Ping Injection Combined with Chemotherapy in Advanced Gastric Cancer: A Systematic Review and Meta-Analysis.Evid Based Complement Alternat Med. 2019 May 19;2019:3821053. doi: 10.1155/2019/3821053. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31236124 Free PMC article. Review.
Cited by
-
Efficacy and Safety of Traditional Chinese Medicines as a Complementary Therapy Combined With Chemotherapy in the Treatment of Gastric Cancer: An Overview of Systematic Reviews and Meta-Analyses.Integr Cancer Ther. 2024 Jan-Dec;23:15347354231225961. doi: 10.1177/15347354231225961. Integr Cancer Ther. 2024. PMID: 38229425 Free PMC article. Review.
-
Effectiveness and safety of combined treatment with herbal medicines and palliative chemotherapy for advanced gastric cancer: A systematic review, and meta-analysis.Integr Med Res. 2025 Mar;14(1):101098. doi: 10.1016/j.imr.2024.101098. Epub 2024 Nov 22. Integr Med Res. 2025. PMID: 39911985 Free PMC article. Review.
-
Herb-drug interactions in oncology: pharmacodynamic/pharmacokinetic mechanisms and risk prediction.Chin Med. 2025 Jul 7;20(1):107. doi: 10.1186/s13020-025-01156-4. Chin Med. 2025. PMID: 40624553 Free PMC article. Review.
-
Yiwei decoction promotes apoptosis of gastric cancer cells through spleen-derived exosomes.Front Pharmacol. 2023 Jun 1;14:1144955. doi: 10.3389/fphar.2023.1144955. eCollection 2023. Front Pharmacol. 2023. PMID: 37324462 Free PMC article.
-
Molecular targets and mechanisms of traditional Chinese medicine combined with chemotherapy for gastric cancer: a meta-analysis and multi-omics approach.Ann Med. 2025 Dec;57(1):2494671. doi: 10.1080/07853890.2025.2494671. Epub 2025 May 3. Ann Med. 2025. PMID: 40317214 Free PMC article.
References
-
- Ajani J. A., Moiseyenko V. M., Tjulandin S., Majlis A., Constenla M., Boni C., et al. (2007). Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: The V-325 study group. J. Clin. Oncol. 22, 3205–3209. 10.1200/JCO.2006.10.4968 - DOI - PubMed
-
- Al-Batran S. E., Hartmann J. T., Probst S., Schmalenberg H., Hollerbach S., Hofheinz R., et al. (2008). Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J. Clin. Oncol. 9, 1435–1442. 10.1200/JCO.2007.13.9378 - DOI - PubMed
-
- Amin A., Hossen M. J., Fu X. Q., Chou J. Y., Wu J. Y., Wang X. Q., et al. (2022). Inhibition of the Akt/NF-κB pathway is involved in the anti-gastritis effects of an ethanolic extract of the rhizome of Atractylodes macrocephala. J. Ethnopharmacol. 293, 115251. 10.1016/j.jep.2022.115251 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

