How do cancer clinicians perceive real-world data and the evidence derived therefrom? Findings from an international survey of the European Organisation for Research and Treatment of Cancer
- PMID: 36091761
- PMCID: PMC9449152
- DOI: 10.3389/fphar.2022.969778
How do cancer clinicians perceive real-world data and the evidence derived therefrom? Findings from an international survey of the European Organisation for Research and Treatment of Cancer
Abstract
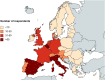
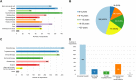
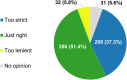
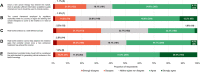
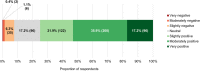
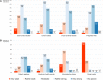
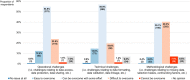
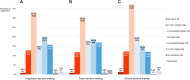
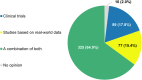
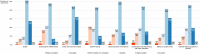
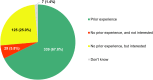
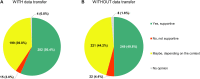
Background: The role of real-world evidence (RWE) in the development of anticancer therapies has been gradually growing over time. Regulators, payers and health technology assessment agencies, spurred by the rise of the precision medicine model, are increasingly incorporating RWE into their decision-making regarding the authorization and reimbursement of novel antineoplastic treatments. However, it remains unclear how this trend is viewed by clinicians in the field. This study aimed to investigate the opinions of these stakeholders with respect to RWE and its suitability for informing regulatory, reimbursement-related and clinical decisions in oncology. Methods: An online survey was disseminated to clinicians belonging to the network of the European Organisation for Research and Treatment of Cancer between May and July 2021. Results: In total, 557 clinicians across 30 different countries participated in the survey, representing 13 distinct cancer domains. Despite seeing the methodological challenges associated with its interpretation as difficult to overcome, the respondents mostly (75.0%) perceived RWE positively, and believed such evidence could be relatively strong, depending on the designs and data sources of the studies from which it is produced. Few (4.6%) saw a future expansion of its influence on decision-makers as a negative evolution. Furthermore, nearly all (94.0%) participants were open to the idea of sharing anonymized or pseudonymized electronic health data of their patients with external parties for research purposes. Nevertheless, most clinicians (77.0%) still considered randomized controlled trials (RCTs) to be the gold standard for generating clinical evidence in oncology, and a plurality (49.2%) thought that RWE cannot fully address the knowledge gaps that remain after a new antitumor intervention has entered the market. Moreover, a majority of respondents (50.7%) expressed that they relied more heavily on RCT-derived evidence than on RWE for their own decision-making. Conclusion: While cancer clinicians have positive opinions about RWE and want to contribute to its generation, they also continue to hold RCTs in high regard as sources of actionable evidence.
Keywords: Europe; cancer; clinicians; oncology; randomized controlled trials; real-world data; real-world evidence; survey.
Copyright © 2022 Saesen, Kantidakis, Marinus, Lacombe and Huys.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Payer perceptions of the use of real-world evidence in oncology-based decision making.J Manag Care Spec Pharm. 2021 Aug;27(8):1096-1105. doi: 10.18553/jmcp.2021.27.8.1096. J Manag Care Spec Pharm. 2021. PMID: 34337998 Free PMC article.
-
The maze of real-world evidence frameworks: from a desert to a jungle! An environmental scan and comparison across regulatory and health technology assessment agencies.J Comp Eff Res. 2024 Sep;13(9):e240061. doi: 10.57264/cer-2024-0061. Epub 2024 Aug 12. J Comp Eff Res. 2024. PMID: 39132748 Free PMC article.
-
Use of real-world evidence for oncology clinical decision making in emerging economies.Future Oncol. 2021 Aug;17(22):2951-2960. doi: 10.2217/fon-2021-0425. Epub 2021 May 28. Future Oncol. 2021. PMID: 34044583 Review.
-
Defining the role of real-world data in cancer clinical research: The position of the European Organisation for Research and Treatment of Cancer.Eur J Cancer. 2023 Jun;186:52-61. doi: 10.1016/j.ejca.2023.03.013. Epub 2023 Mar 21. Eur J Cancer. 2023. PMID: 37030077
-
Use of Real-World Evidence in US Payer Coverage Decision-Making for Next-Generation Sequencing-Based Tests: Challenges, Opportunities, and Potential Solutions.Value Health. 2020 May;23(5):540-550. doi: 10.1016/j.jval.2020.02.001. Epub 2020 Mar 26. Value Health. 2020. PMID: 32389218 Free PMC article. Review.
Cited by
-
Cytochrome P450 inhibitor/inducer treatment patterns among patients in the United States with advanced ovarian cancer who were prescribed or were eligible for poly(adenosine diphosphate [ADP]-ribose) polymerase inhibitors in the first-line maintenance setting.Gynecol Oncol Rep. 2024 Feb 7;51:101332. doi: 10.1016/j.gore.2024.101332. eCollection 2024 Feb. Gynecol Oncol Rep. 2024. PMID: 38362364 Free PMC article.
-
Positive correlation between persistence of medical nutrition therapy and overall survival in patients with head and neck cancer.Pathol Oncol Res. 2024 Mar 15;30:1611664. doi: 10.3389/pore.2024.1611664. eCollection 2024. Pathol Oncol Res. 2024. PMID: 38559567 Free PMC article.
-
Harnessing the Potential of Real-World Evidence in the Treatment of Colorectal Cancer: Where Do We Stand?Curr Treat Options Oncol. 2024 Apr;25(4):405-426. doi: 10.1007/s11864-024-01186-4. Epub 2024 Feb 17. Curr Treat Options Oncol. 2024. PMID: 38367182 Free PMC article. Review.
-
Real World Data Studies of Antineoplastic Drugs: How Can They Be Improved to Steer Everyday Use in the Clinic?Pragmat Obs Res. 2023 Sep 6;14:95-100. doi: 10.2147/POR.S395959. eCollection 2023. Pragmat Obs Res. 2023. PMID: 37701044 Free PMC article. Review.
-
Real-world data in oncology: a questionnaire-based analysis of the academic research landscape examining the policies and experiences of the cancer cooperative groups.ESMO Open. 2023 Apr;8(2):100878. doi: 10.1016/j.esmoop.2023.100878. Epub 2023 Feb 21. ESMO Open. 2023. PMID: 36822113 Free PMC article.
References
LinkOut - more resources
Full Text Sources

