A novel form of docetaxel polymeric micelles demonstrates anti-tumor and ascites-inhibitory activities in animal models as monotherapy or in combination with anti-angiogenic agents
- PMID: 36091776
- PMCID: PMC9449419
- DOI: 10.3389/fphar.2022.964076
A novel form of docetaxel polymeric micelles demonstrates anti-tumor and ascites-inhibitory activities in animal models as monotherapy or in combination with anti-angiogenic agents
Abstract
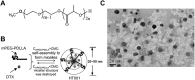
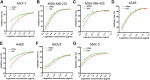
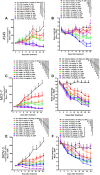
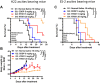
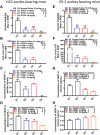
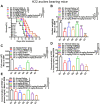
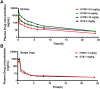
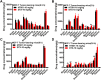
Malignant ascites (MA) is caused by intraperitoneal spread of solid tumor cells and results in a poor quality of life. Chemotherapy is a common first-line treatment for patients with MA. Taxotere ® (DTX) is widely used in solid tumor therapies. However, the low water solubility and side effects caused by additives in the formulation restrict the clinical application of docetaxel. HT001 is a clinical stage docetaxel micelle developed to overcome the solubility issue with improved safety profiles. To support clinical development and expand clinical application of HT001, this study used in vitro and in vivo approaches to investigate the anti-tumor effects of HT001 when applied as monotherapy or in combination with anti-angiogenic agents. HT001 demonstrated comparable anti-proliferative activities as docetaxel in a broad range of cancer cell lines in vitro. Furthermore, HT001 suppressed tumor growth in a dose-dependent manner in A549, MCF-7, and SKOV-3 xenograft tumor mouse models in vivo. In a hepatocellular carcinoma H22 malignant ascites-bearing mouse model, HT001 presented a dose-dependent inhibition of ascites production, prolonged animal survival, and reduced VEGF levels. When dosed at 20 mg/kg, the HT001-treated group exhibited curative results, with no ascites formation in 80% of mice at the end of the study while all the mice in the vehicle control group succumbed. Similar results were obtained in HT001 treatment of mice bearing malignant ascites produced by human ovarian cancer ES-2 cells. Notably, the combination of HT001 with Endostar not only significantly reduced ascites production but also prolonged survival of H22 ascites-bearing mice. HT001 showed similar PK and tissue distribution profiles as DTX in non-rodent hosts. Collectively, these results demonstrate potent anti-tumor activity of HT001 in multiple solid tumor models or malignant ascites models, and reveal synergistic effects with anti-angiogenic agents, supporting the clinical development and clinical expansion plans for HT001.
Keywords: anti-angiogenic agents; anti-tumor effects; ascites-inhibitory activities; combination therapy; docetaxel polymeric micelles.
Copyright © 2022 Guo, Qin, Xue, Yang, Zhang, Zhu, Ye, Tang and Yang.
Conflict of interest statement
Authors LG, XQ, LX, SZ, GY, RT, and WY were employed by the company Jiangsu Simcere Pharmaceutical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bhaskari J., Krishnamoorthy L. (2015). Impact of ascites and plasma levels of VEGF-A in epithelial ovarian cancers. J. Clin. Cell. Immunol. 6, 4. 10.4172/2155-9899.1000353 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

