Pharmacokinetic assessment of vancomycin in critically ill patients and nephrotoxicity prediction using individualized pharmacokinetic parameters
- PMID: 36091788
- PMCID: PMC9449142
- DOI: 10.3389/fphar.2022.912202
Pharmacokinetic assessment of vancomycin in critically ill patients and nephrotoxicity prediction using individualized pharmacokinetic parameters
Abstract
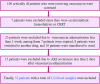
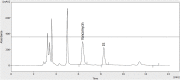
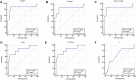
Introduction: Therapeutic drug monitoring (TDM) and pharmacokinetic assessments of vancomycin would be essential to avoid vancomycin-associated nephrotoxicity and obtain optimal therapeutic and clinical responses. Different pharmacokinetic parameters, including trough concentration and area under the curve (AUC), have been proposed to assess the safety and efficacy of vancomycin administration. Methods: Critically ill patients receiving vancomycin at Nemazee Hospital were included in this prospective study. Four blood samples at various time intervals were taken from each participated patient. Vancomycin was extracted from plasma samples and analyzed using a validated HPLC method. Results: Fifty-three critically ill patients with a total of 212 blood samples from June 2019 to June 2021 were included in this study. There was a significant correlation between baseline GFR, baseline serum creatinine, trough and peak concentrations, AUCτ, AUC24h, Cl, and Vd values with vancomycin-induced AKI. Based on trough concentration values, 66% of patients were under-dosed (trough concentration <15 μg/ml) and 18.9% were over-dosed (trough concentration ≥20 μg/ml). Also, based on AUC24h values, about 52.2% were under-dosed (AUC24h < 400 μg h/ml), and 21.7% were over-dosed (AUC24h > 600 μg h/ml) that emphasizes on the superiority of AUC-based monitoring approach for TDM purposes to avoid nephrotoxicity occurrence. Conclusion: The AUC-based monitoring approach would be superior in terms of nephrotoxicity prediction. Also, to avoid vancomycin-induced AKI, trough concentration and AUCτ values should be maintained below the cut-off points.
Keywords: AUC of intervals (AUCτ); ROC curves; critically ill patients; cut-off point; nephrotoxicity; pharmacokinetic parameters; therapeutic drug monitoring (TDM); vancomycin.
Copyright © 2022 Ghasemiyeh, Vazin, Zand, Haem, Karimzadeh, Azadi, Masjedi, Sabetian, Nikandish and Mohammadi-Samani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Investigation on the association between Osteopontin and Apolipoprotein E gene polymorphisms and vancomycin-induced acute kidney injury: A pharmacokinetic/pharmacogenetic study in critically ill patients.Gene. 2025 Jun 10;952:149386. doi: 10.1016/j.gene.2025.149386. Epub 2025 Mar 11. Gene. 2025. PMID: 40081681
-
Assessing Nephrotoxicity Associated With Different Vancomycin Dosing Modalities in Obese Patients at a Community Hospital.Hosp Pharm. 2022 Aug;57(4):532-539. doi: 10.1177/00185787211055791. Epub 2021 Dec 14. Hosp Pharm. 2022. PMID: 35898248 Free PMC article.
-
Reduced nephrotoxicity with vancomycin therapeutic drug monitoring guided by area under the concentration-time curve against a trough 15-20 μg/mL concentration.Int J Antimicrob Agents. 2020 Oct;56(4):106109. doi: 10.1016/j.ijantimicag.2020.106109. Epub 2020 Jul 25. Int J Antimicrob Agents. 2020. PMID: 32721597
-
Area-Under-Curve-Guided Versus Trough-Guided Monitoring of Vancomycin and Its Impact on Nephrotoxicity: A Systematic Review and Meta-Analysis.Ther Drug Monit. 2023 Aug 1;45(4):519-532. doi: 10.1097/FTD.0000000000001075. Epub 2023 Jan 10. Ther Drug Monit. 2023. PMID: 36728329 Free PMC article.
-
The monitoring of vancomycin: a systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing.BMC Infect Dis. 2021 Feb 6;21(1):153. doi: 10.1186/s12879-021-05858-6. BMC Infect Dis. 2021. PMID: 33549035 Free PMC article.
Cited by
-
Development of a Machine Learning-Based Predictive Model for Postoperative Delirium in Older Adult Intensive Care Unit Patients: Retrospective Study.J Med Internet Res. 2025 Jun 19;27:e67258. doi: 10.2196/67258. J Med Internet Res. 2025. PMID: 40537091 Free PMC article.
-
Vancomycin levels for Bayesian dose-optimization in critical care: a prospective cohort study.Front Med (Lausanne). 2025 Jul 22;12:1575224. doi: 10.3389/fmed.2025.1575224. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40766073 Free PMC article.
-
A Deep Learning-Based Approach for Prediction of Vancomycin Treatment Monitoring: Retrospective Study Among Patients With Critical Illness.JMIR Form Res. 2024 Mar 8;8:e45202. doi: 10.2196/45202. JMIR Form Res. 2024. PMID: 38152042 Free PMC article.
-
A Multicenter Randomized Controlled Study on Pharmacokinetic-Guided Vancomycin Use in Children With Severe Infections.Clin Transl Sci. 2025 Aug;18(8):e70309. doi: 10.1111/cts.70309. Clin Transl Sci. 2025. PMID: 40704610 Free PMC article. Clinical Trial.
-
Volume of distribution as an early predictor of vancomycin-induced AKI in critically ill patients.Acta Biomed. 2023 Oct 17;94(5):e2023185. doi: 10.23750/abm.v94i5.14158. Acta Biomed. 2023. PMID: 37850781 Free PMC article. No abstract available.
References
-
- Drugbank (2022). Vancomycin. Available at: https://go.drugbank.com/drugs/DB00512 . (Accessed April, 2022).
LinkOut - more resources
Full Text Sources

