How zoledronic acid improves osteoporosis by acting on osteoclasts
- PMID: 36091799
- PMCID: PMC9452720
- DOI: 10.3389/fphar.2022.961941
How zoledronic acid improves osteoporosis by acting on osteoclasts
Abstract
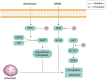
Osteoporosis is called a silent disease, because it is difficult to detect until comprehensive examinations for osteoporosis are performed or osteoporotic fractures occur. Zoledronic acid is currently the first-line anti-osteoporotic drug, with good efficacy and treatment compliance. A major advantage of zoledronic acid is that intravenous zoledronic acid often guarantees a therapeutic effect for up to 1 year after infusion. The reasons why zoledronic acid is effective in improving osteoporosis are that it can inhibit osteoclast differentiation and induce osteoclast apoptosis, thus suppressing bone resorption and increasing bone density. The story between zoledronic acid and osteoclasts has been written long time ago. Both the canonical receptor activator of the receptor activator of nuclear factor-κB ligand (RANKL) pathway and the non-canonical Wnt pathway are the main pathways by which zoledronic acid inhibits osteoclast differentiation. Farnesyl pyrophosphate synthase (FPPS), reactive oxygen species (ROS), and ferroptosis that was first proposed in 2012, are all considered to be closely associated with zoledronic acid-induced osteoclast apoptosis. Here, we provide a brief review of the recent progress on the study of zoledronic acid and osteoclasts, and hope to elaborate how zoledronic acid improves osteoporosis by acting on osteoclasts.
Keywords: apoptosis; differentiation; osteoclasts; osteoporosis; signaling; signaling pathways; zoledronic acid.
Copyright © 2022 Wang, Zhan, Yan and Hao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Various pathways of zoledronic acid against osteoclasts and bone cancer metastasis: a brief review.BMC Cancer. 2020 Nov 3;20(1):1059. doi: 10.1186/s12885-020-07568-9. BMC Cancer. 2020. PMID: 33143662 Free PMC article. Review.
-
Zoledronic acid inhibits RANK expression and migration of osteoclast precursors during osteoclastogenesis.Naunyn Schmiedebergs Arch Pharmacol. 2011 Mar;383(3):297-308. doi: 10.1007/s00210-010-0596-4. Epub 2011 Jan 12. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21225243
-
Activation of p38 MAPK-regulated Bcl-xL signaling increases survival against zoledronic acid-induced apoptosis in osteoclast precursors.Bone. 2014 Oct;67:166-74. doi: 10.1016/j.bone.2014.07.003. Epub 2014 Jul 9. Bone. 2014. PMID: 25016096
-
Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha.Free Radic Biol Med. 2006 May 1;40(9):1483-93. doi: 10.1016/j.freeradbiomed.2005.10.066. Epub 2005 Dec 9. Free Radic Biol Med. 2006. PMID: 16632109
-
Cell-based assay strategy for identification of motif-specific RANK signaling pathway inhibitors.Assay Drug Dev Technol. 2006 Aug;4(4):473-82. doi: 10.1089/adt.2006.4.473. Assay Drug Dev Technol. 2006. PMID: 16945019 Review.
Cited by
-
Role of SIRT3 in bone homeostasis and its application in preventing and treating bone diseases.Front Pharmacol. 2023 Dec 20;14:1248507. doi: 10.3389/fphar.2023.1248507. eCollection 2023. Front Pharmacol. 2023. PMID: 38192409 Free PMC article. Review.
-
Zoledronate Therapy in Osteogenesis Imperfecta: Perspectives in Indonesia Tertiary Hospital.J Bone Metab. 2024 Nov;31(4):290-295. doi: 10.11005/jbm.24.767. Epub 2024 Nov 4. J Bone Metab. 2024. PMID: 39496298 Free PMC article.
-
A novel shockwave-driven nanomotor composite microneedle transdermal delivery system for the localized treatment of osteoporosis: a basic science study.Int J Surg. 2024 Oct 1;110(10):6243-6256. doi: 10.1097/JS9.0000000000001280. Int J Surg. 2024. PMID: 39259829 Free PMC article.
-
Effects of Combined Application of Percutaneous Vertebroplasty and Zoledronic Acid on Bone Mineral Density, Bone Metabolism, NPY and PGE2 in Elderly Patients with Osteoporotic Lumbar Vertebral Compression Fracture.J Musculoskelet Neuronal Interact. 2024 Jun 1;24(2):192-199. J Musculoskelet Neuronal Interact. 2024. PMID: 38826002 Free PMC article.
-
Evaluate the renal system damage caused by zoledronic acid: a comprehensive analysis of adverse events from FAERS.BMC Cancer. 2024 Dec 18;24(1):1520. doi: 10.1186/s12885-024-13284-5. BMC Cancer. 2024. PMID: 39695477 Free PMC article.
References
-
- Abdelmagid S. M., Sondag G. R., Moussa F. M., Belcher J. Y., Yu B., Stinnett H., et al. (2015). Mutation in osteoactivin promotes receptor activator of NFκB ligand (RANKL)-mediated osteoclast differentiation and survival but inhibits osteoclast function. J. Biol. Chem. 290 (33), 20128–20146. 10.1074/jbc.M114.624270 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

