Rhein ameliorates transverse aortic constriction-induced cardiac hypertrophy via regulating STAT3 and p38 MAPK signaling pathways
- PMID: 36091816
- PMCID: PMC9459036
- DOI: 10.3389/fphar.2022.940574
Rhein ameliorates transverse aortic constriction-induced cardiac hypertrophy via regulating STAT3 and p38 MAPK signaling pathways
Abstract
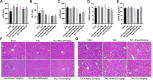
The progression from compensatory hypertrophy to heart failure is difficult to reverse, in part due to extracellular matrix fibrosis and continuous activation of abnormal signaling pathways. Although the anthraquinone rhein has been examined for its many biological properties, it is not clear whether it has therapeutic value in the treatment of cardiac hypertrophy and heart failure. In this study, we report for the first time that rhein can ameliorate transverse aortic constriction (TAC)-induced cardiac hypertrophy and other cardiac damage in vivo and in vitro. In addition, rhein can reduce cardiac hypertrophy by attenuating atrial natriuretic peptide, brain natriuretic peptide, and β-MHC expression; cardiac fibrosis; and ERK phosphorylation and transport into the nucleus. Furthermore, the inhibitory effect of rhein on myocardial hypertrophy was similar to that of specific inhibitors of STAT3 and ERK signaling. In addition, rhein at therapeutic doses had no significant adverse effects or toxicity on liver and kidney function. We conclude that rhein reduces TAC-induced cardiac hypertrophy via targeted inhibition of the molecular function of ERK and downregulates STAT3 and p38 MAPK signaling. Therefore, rhein might be a novel and effective agent for treating cardiac hypertrophy and other cardiovascular diseases.
Keywords: ERK phosphorylation; STAT; cardiac fibrosis; cardiac hypertrophy; p38 MAPK; rhein; transverse aortic constriction.
Copyright © 2022 Li, Xu, Zhang, Chen, Liu, Yang, Li, Luo, Liu, Tang and Shan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

