Toxicological safety evaluation of Qin-Zhi-Zhu-Dan formula in rats during the treatment and recovery periods
- PMID: 36091824
- PMCID: PMC9453232
- DOI: 10.3389/fphar.2022.987997
Toxicological safety evaluation of Qin-Zhi-Zhu-Dan formula in rats during the treatment and recovery periods
Abstract
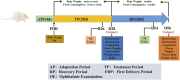
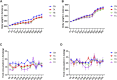
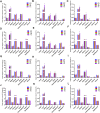
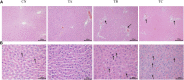
Background: Qinzhi Zhudan Formula (QZZD), optimized from Angong Niuhuang Wan, consists of Radix Scutellariae, Fructus Gardeniae and Pulvis Fellis Suis. We had investigated the neuroprotective effects of QZZD and its active components, and demonstrated that it could treat cerebral ischemia and dementia through multiple pathways and mechanisms. Nevertheless, toxicological data on this formula still remains limited. In the study, we sought to examine the toxicological effects of QZZD during the treatment and recovery periods. Methods: We investigated potential toxicities of QZZD in Sprague-Dawley (SD) rats via 28-day gavage administration. SD rats were randomly divided into control group and treatment groups of A (0.5 g/kg/d QZZD), B (1.5 g/kg/d QZZD), and C (5.0 g/kg/d QZZD). The 56-day course includes treatment period (administration with water or QZZD once a day for 28 consecutive days) and recovery period (28 days). The rats received daily monitoring of general signs of toxicity and mortality, as well as weekly determination of body weight and food consumption. Moreover, the complete blood cell count, biochemistry, coagulation, and urine indicators, organ weights, and histopathological report were analyzed respectively at the end of the treatment and recovery periods. Results: There was no death related to the active pharmaceutical ingredients of QZZD during the treatment period. The maximum no observed adverse effect level (NOAEL) was 0.5 g/kg/d, which is approximately 16.7 times of the equivalent dose of clinical dose in rats. In group TB (1.5 g/kg/d QZZD) and TC (5.0 g/kg/d QZZD), there were adverse effects of blue coloring of tail skin, weight loss, a significant increase of total bilirubin (TBIL), blackening of liver and kidney in gross examination, hyperplasia of bile duct and karyomegaly of hepatocytes in histopathological examination. Besides, in females rats, the food consumption was reduced, while in male rats, there was decrease in triglycerides (TG) and slight increase in white blood cell (WBC) count and neutrophils. In group TC (5.0 g/kg/d QZZD), the indicators of red blood cell (RBC) count, hemoglobin (HGB) and hematocrit (HCT) were decreased slightly, while the platelet count (PLT) was increased. However, these changes were not considered to be toxicologically significant because they resolved during the recovery period. Conclusion: Overall, QZZD exhibited a good safety profile. The maximum no observed adverse effect level was 0.5 g/kg/d, and no target organs toxicity were identified. The present findings might confirm the safety of QZZD in clinical practices.
Keywords: biochemical; gardenia extract; organ coefficient; pulvis fellis suis; reversibility study; scutellaria baicalensis extract; sub-chronic toxicity.
Copyright © 2022 Xu, Chen, Zhang, Liu, Chen, Sun, Ni, Kang, Shang, Wang, Cheng and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Network pharmacology analysis reveals neuroprotective effects of the Qin-Zhi-Zhu-Dan Formula in Alzheimer's disease.Front Neurosci. 2022 Oct 20;16:943400. doi: 10.3389/fnins.2022.943400. eCollection 2022. Front Neurosci. 2022. PMID: 36340795 Free PMC article.
-
Qinzhizhudan formula dampens inflammation in microglia polarization of vascular dementia rats by blocking MyD88/NF-κB signaling pathway: Through integrating network pharmacology and experimental validation.J Ethnopharmacol. 2024 Jan 10;318(Pt A):116769. doi: 10.1016/j.jep.2023.116769. Epub 2023 Jul 1. J Ethnopharmacol. 2024. PMID: 37400007
-
NTP Toxicology and Carcinogenesis Studies of Coumarin (CAS No. 91-64-5) in F344/N Rats and B6C3F1 Mice (Gavage Studies).Natl Toxicol Program Tech Rep Ser. 1993 Sep;422:1-340. Natl Toxicol Program Tech Rep Ser. 1993. PMID: 12616289
-
Toxicology and carcinogenesis studies of androstenedione (CAS No. 63-05-8) in F344/N rats and B6C3F1 mice (gavage studies).Natl Toxicol Program Tech Rep Ser. 2010 Sep;(560):1, 7-31,33-171 passim. Natl Toxicol Program Tech Rep Ser. 2010. PMID: 21037592 Review.
-
Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin.Int J Toxicol. 2007;26 Suppl 1:3-106. doi: 10.1080/10915810601163939. Int J Toxicol. 2007. PMID: 17365137 Review.
Cited by
-
Network pharmacology analysis reveals neuroprotective effects of the Qin-Zhi-Zhu-Dan Formula in Alzheimer's disease.Front Neurosci. 2022 Oct 20;16:943400. doi: 10.3389/fnins.2022.943400. eCollection 2022. Front Neurosci. 2022. PMID: 36340795 Free PMC article.
-
Biocompatibility and sub-chronic toxicity studies of phlorotannin/polycaprolactone coated trachea tube for advancing medical device applications.Sci Rep. 2024 Feb 16;14(1):3945. doi: 10.1038/s41598-024-54684-8. Sci Rep. 2024. PMID: 38365854 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

