Recent advances of IDH1 mutant inhibitor in cancer therapy
- PMID: 36091829
- PMCID: PMC9449373
- DOI: 10.3389/fphar.2022.982424
Recent advances of IDH1 mutant inhibitor in cancer therapy
Abstract
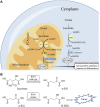
Isocitrate dehydrogenase (IDH) is the key metabolic enzyme that catalyzes the conversion of isocitrate to α-ketoglutarate (α-KG). Two main types of IDH1 and IDH2 are present in humans. In recent years, mutations in IDH have been observed in several tumors, including glioma, acute myeloid leukemia, and chondrosarcoma. Among them, the frequency of IDH1 mutations is higher than IDH2. IDH1 mutations have been shown to increase the conversion of α-KG to 2-hydroxyglutarate (2-HG). IDH1 mutation-mediated accumulation of 2-HG leads to epigenetic dysregulation, altering gene expression, and impairing cell differentiation. A rapidly emerging therapeutic approach is through the development of small molecule inhibitors targeting mutant IDH1 (mIDH1), as evidenced by the recently approved of the first selective IDH1 mutant inhibitor AG-120 (ivosidenib) for the treatment of IDH1-mutated AML. This review will focus on mIDH1 as a therapeutic target and provide an update on IDH1 mutant inhibitors in development and clinical trials.
Keywords: 2-HG; hypermethylation; isocitrate dehydrogenase mutation; mIDH1 inhibitors; natural product.
Copyright © 2022 Tian, Zhang, Wang, Jin, Wang, Guo, Tang and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
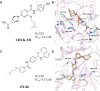
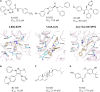
Similar articles
-
Development of Novel Therapeutics Targeting Isocitrate Dehydrogenase Mutations in Cancer.Curr Top Med Chem. 2018;18(6):505-524. doi: 10.2174/1568026618666180518091144. Curr Top Med Chem. 2018. PMID: 29773061 Review.
-
IDH mutations in cancer and progress toward development of targeted therapeutics.Ann Oncol. 2016 Apr;27(4):599-608. doi: 10.1093/annonc/mdw013. Ann Oncol. 2016. PMID: 27005468 Review.
-
Crystal structures of pan-IDH inhibitor AG-881 in complex with mutant human IDH1 and IDH2.Biochem Biophys Res Commun. 2018 Sep 18;503(4):2912-2917. doi: 10.1016/j.bbrc.2018.08.068. Epub 2018 Aug 18. Biochem Biophys Res Commun. 2018. PMID: 30131249
-
Preclinical Drug Metabolism, Pharmacokinetic, and Pharmacodynamic Profiles of Ivosidenib, an Inhibitor of Mutant Isocitrate Dehydrogenase 1 for Treatment of Isocitrate Dehydrogenase 1-Mutant Malignancies.Drug Metab Dispos. 2021 Oct;49(10):870-881. doi: 10.1124/dmd.120.000234. Epub 2021 Jul 28. Drug Metab Dispos. 2021. PMID: 34321251
-
Reviewing the IDH1 Mutation-Mediated Mechanism of Drug Resistance and Revisiting Its Overcoming Strategies.Chem Biol Drug Des. 2025 Apr;105(4):e70102. doi: 10.1111/cbdd.70102. Chem Biol Drug Des. 2025. PMID: 40183465 Review.
Cited by
-
Integrated Gene Expression Data-Driven Identification of Molecular Signatures, Prognostic Biomarkers, and Drug Targets for Glioblastoma.Biomed Res Int. 2024 Aug 16;2024:6810200. doi: 10.1155/2024/6810200. eCollection 2024. Biomed Res Int. 2024. PMID: 39184354 Free PMC article.
-
Grade 2, 3 and Dedifferentiated Chondrosarcomas: A Comparative Study of Isocitrate Dehydrogenase-Mutant and Wild-Type Tumors with Implications for Prognosis and Therapy.Cancers (Basel). 2024 Jan 5;16(2):247. doi: 10.3390/cancers16020247. Cancers (Basel). 2024. PMID: 38254737 Free PMC article.
-
Advancing the Management of Skull Base Chondrosarcomas: A Systematic Review of Targeted Therapies.J Pers Med. 2024 Feb 28;14(3):261. doi: 10.3390/jpm14030261. J Pers Med. 2024. PMID: 38541003 Free PMC article. Review.
-
3D-QSAR, Scaffold Hopping, Virtual Screening, and Molecular Dynamics Simulations of Pyridin-2-one as mIDH1 Inhibitors.Int J Mol Sci. 2024 Jul 6;25(13):7434. doi: 10.3390/ijms25137434. Int J Mol Sci. 2024. PMID: 39000539 Free PMC article.
-
Preparation and Preclinical Evaluation of 18F-Labeled Olutasidenib Derivatives for Non-Invasive Detection of Mutated Isocitrate Dehydrogenase 1 (mIDH1).Molecules. 2024 Aug 21;29(16):3939. doi: 10.3390/molecules29163939. Molecules. 2024. PMID: 39203017 Free PMC article.
References
-
- Abou-Alfa G. K., Macarulla T., Javle M. M., Kelley R. K., Lubner S. J., Adeva J., et al. (2020). Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet. Oncol. 21 (6), 796–807. 10.1016/s1470-2045(20)30157-1 - DOI - PMC - PubMed
-
- Caferro T. R., Cho Y. S., Costales A. Q., Lei H., Lenoir F., Levell J. R., et al. (2015). 3-pyrimidin-4-yl-oxazolidin-2-ones as inhibitors of mutant IDH. US.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

