Insensitivity to T790M mutation? A pooled analysis of outcomes following osimertinib for the treatment of NSCLC patients harboring uncommon epidermal growth factor receptor mutation
- PMID: 36091840
- PMCID: PMC9458881
- DOI: 10.3389/fphar.2022.986962
Insensitivity to T790M mutation? A pooled analysis of outcomes following osimertinib for the treatment of NSCLC patients harboring uncommon epidermal growth factor receptor mutation
Abstract
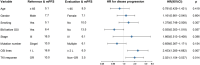
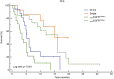
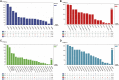
Background: In this pooled analysis, the aim was to investigate the clinicopathological characteristics of patients with uncommon epidermal growth factor receptor (EGFR) (ucm-EGFRms) along with their treatment responses and survival following osimertinib treatment. Methods: Univariate chi-square analysis was conducted to analyze the correlation between clinical characteristics, EGFR mutation type, and treatment response, and the Kaplan-Meier method was applied for survival analysis. Univariate logistic regression model and Cox proportional hazards model were performed to compare the efficacy and prognosis in subgroup analysis. Results: Seventy-two NSCLC patients in total were included in this pooled analysis. The objective response rate (ORR) for osimertinib treatment was 57.0%, with a median PFS of 7.1 months. Twenty-eight patients received osimertinib as first-line therapy with an ORR of 67.9%, which was higher than that in patients who received osimertinib as second- or later-line therapy, and their response rate was 50%, nevertheless, no statistically significant differences were found (p = 0.139). However, patients who received first-line osimertinib showed a more significant PFS benefit than those who received second- or later-line therapy (mPFS: 16.8 months vs 6.0 months HR: 2.453, 95%CI: 1.285-4.682, p =0.004). Subgroup analysis showed that patients with a single, non-ex20ins, ucm-EGFRm displayed a superior efficacy advantage and favorable survival benefit following osimertinib treatment, with an ORR of 68.8% and an mPFS at 15.1 months. By contrast, patients with a multiple ucm-EGFRm that contain T790M exhibited the worst outcome of osimertinib treatment, with an ORR of 47.6% and an mPFS of only 3.6 months, respectively. Conclusion: Patients with um-EGFRms exhibit favorable but inconsistent responses and survival outcomes following osimertinib treatment, which is closely related to the mutation pattern and cooccurring partner mutant genes. Administering osimertinib for the treatment of patients with um-EGFRm might be considered an effective treatment option in some circumstances.
Keywords: EGFR; Efficacy; NSCLC6; Prognosis; osimertinib; uncommon.
Copyright © 2022 Hu, Wang, Wang, Zhao, Wang and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ahn M. J., Chiu C. H., Cheng Y., Han J. Y., Goldberg S. B., Greystoke A., et al. (2020). Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: The AURA leptomeningeal metastases analysis. J. Thorac. Oncol. 15, 637–648. 10.1016/j.jtho.2019.12.113 - DOI - PubMed
-
- Ballard P., Yates J. W., Yang Z., Kim D. W., Yang J. C., Cantarini M., et al. (2016). Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin. Cancer Res. 22, 5130–5140. 10.1158/1078-0432.Ccr-16-0399 - DOI - PubMed
-
- Cheema P., Passaro A., Martin C., Tiseo M., Park K., Chang G., et al. (2019). P2.14-59 osimertinib in EGFR T790M advanced NSCLC: Analysis of uncommon/complex EGFR mutations in a real-world study (ASTRIS). J. Thorac. Oncol. 14, S854. Abstract retrieved from International Association for the Study of Lung Cancer (IASLC) (Poster sessions. P2.14-59). 10.1016/j.jtho.2019.08.1844 - DOI
-
- Cho J. H., Lim S. H., An H. J., Kim K. H., Park K. U., Kang E. J., et al. (2020). Osimertinib for patients with non-small-cell lung cancer harboring uncommon EGFR mutations: A multicenter, open-label, phase II trial (KCSG-LU15-09). J. Clin. Oncol. 38, 488–495. 10.1200/jco.19.00931 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

