Modeling titanium dioxide nanostructures for photocatalysis and photovoltaics
- PMID: 36091912
- PMCID: PMC9400622
- DOI: 10.1039/d2sc02872g
Modeling titanium dioxide nanostructures for photocatalysis and photovoltaics
Abstract
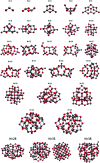
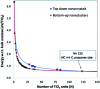
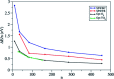
Heterogenous photocatalysis is regarded as a holy grail in relation to the energy and environmental issues with which our society is currently struggling. In this context, the characterization of titanium dioxide nanostructures and the relationships between structural/electronic parameters and chemical/physical-chemical properties is a primary target, whose achievement is in high demand. Theoretical simulations can strongly support experiments to reach this goal. While the bulk and surface properties of TiO2 materials are quite well understood, the field of nanostructures still presents a few unexplored areas. Here we consider possible approaches for the modeling of reduced and extended TiO2 nanostructures, and we review the main outcomes of the investigation of the structural, electronic, and optical properties of TiO2 nanoparticles and their relationships with the size, morphology, and shape of the particles. Further investigations are highly desired to fill the gaps still remaining and to allow improvements in the efficiencies of these materials for photocatalytic and photovoltaic applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
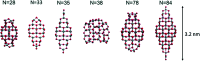


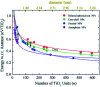
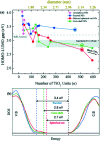



References
-
- Mills A. LeHunte S. J. Photochem. Photobiol. Chem. 1997;108:1–35. doi: 10.1016/S1010-6030(97)00118-4. - DOI
-
- Carp O. Huisman C. L. Reller A. Prog. Solid State Chem. 2004;32:33–177. doi: 10.1016/j.progsolidstchem.2004.08.001. - DOI
-
- Ni M. Leung M. K. H. Leung D. Y. C. Sumathy K. Renew. Sustain. Energy Rev. 2007;11:401–425. doi: 10.1016/j.rser.2005.01.009. - DOI
-
- O'Regan B. Grätzel M. Nature. 1991;353:737–740. doi: 10.1038/353737a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources

