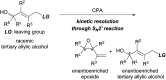
Kinetic resolution of racemic tertiary allylic alcohols through SN2' reaction using a chiral bisphosphoric acid/silver(i) salt co-catalyst system
- PMID: 36091917
- PMCID: PMC9400685
- DOI: 10.1039/d2sc03052g
Kinetic resolution of racemic tertiary allylic alcohols through SN2' reaction using a chiral bisphosphoric acid/silver(i) salt co-catalyst system
Abstract
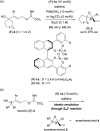
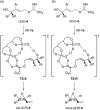
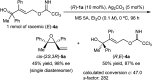
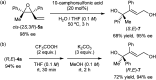
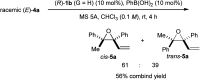
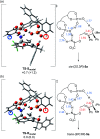
A highly efficient kinetic resolution (KR) of racemic tertiary allylic alcohols was achieved through an intramolecular allylic substitution reaction using a co-catalyst system composed of chiral bisphosphoric acid and silver carbonate. This reaction afforded enantioenriched diene monoepoxides along with the recovery of tertiary allylic alcohols in a highly enantioselective manner, realizing an extremely high s-factor in most cases. The present method provides a new access to enantioenriched tertiary allylic alcohols, multifunctional compounds that are applicable for further synthetic manipulations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Kinetic Resolution of Tertiary 2-Alkoxycarboxamido-Substituted Allylic Alcohols by Chiral Phosphoric Acid Catalyzed Intramolecular Transesterification.Angew Chem Int Ed Engl. 2019 Jul 22;58(30):10315-10319. doi: 10.1002/anie.201905034. Epub 2019 Jun 25. Angew Chem Int Ed Engl. 2019. PMID: 31134717
-
Kinetic Resolution of Tertiary Allylic Alcohols: Highly Enantioselective Access to Cyclic Ethers Bearing an α-Tetrasubstituted Stereocenter.Org Lett. 2021 May 21;23(10):3949-3954. doi: 10.1021/acs.orglett.1c01110. Epub 2021 Apr 30. Org Lett. 2021. PMID: 33929194
-
Highly selective palladium catalyzed kinetic resolution and enantioselective substitution of racemic allylic carbonates with sulfur nucleophiles: asymmetric synthesis of allylic sulfides, allylic sulfones, and allylic alcohols.Chemistry. 2003 Sep 5;9(17):4202-21. doi: 10.1002/chem.200204657. Chemistry. 2003. PMID: 12953206
-
Tandem reactions for streamlining synthesis: enantio- and diastereoselective one-pot generation of functionalized epoxy alcohols.Acc Chem Res. 2008 Aug;41(8):883-93. doi: 10.1021/ar800006h. Acc Chem Res. 2008. PMID: 18710197 Free PMC article. Review.
-
Advances in asymmetric oxidative kinetic resolution of racemic secondary alcohols catalyzed by chiral Mn(III) salen complexes.Chirality. 2017 Dec;29(12):798-810. doi: 10.1002/chir.22768. Epub 2017 Sep 29. Chirality. 2017. PMID: 28963733 Review.
References
-
- Quaternary Stereocenters, Challenges and Solutions for Organic Synthesis, ed. J. Christoffers and A. Baro, Wiley-VCH, Weinheim, 2005
- Science of Synthesis: Stereoselective Synthesis Vol. 2: Stereoselective Reactions of Carbonyl and Imino Groups, ed. G. A. Molander, P. A. Evans and J. G. de Vries, Georg Thieme Verlag KG: New York, 2010
-
- Kagan H. B. and Fiaud J. C. in Topics in Stereochemistry, ed. E. L. Eliel and S. H. Wilen, John Wiley & Sons, New York, 1988, vol. 18, pp. 249–330
- Keith J. M. Larrow J. F. Jacobsen E. N. Adv. Synth. Catal. 2001;343:5–26. doi: 10.1002/1615-4169(20010129)343:1<5::AID-ADSC5>3.0.CO;2-I. - DOI
- Vedejs E. Jure M. Angew. Chem., Int. Ed. 2005;44:3974–4001. doi: 10.1002/anie.200460842. - DOI - PubMed
-
-
For a recent review of non-enzymatic catalytic KR of tertiary alcohols, see:
- Ding B. Xue Q. Jiaa S. Cheng H.-G. Zhou Q. Synthesis. 2022;54:1721–1732. doi: 10.1055/a-1712-0912. - DOI
-
LinkOut - more resources
Full Text Sources

