Immunogenicity assessment of AAV-based gene therapies: An IQ consortium industry white paper
- PMID: 36092368
- PMCID: PMC9418752
- DOI: 10.1016/j.omtm.2022.07.018
Immunogenicity assessment of AAV-based gene therapies: An IQ consortium industry white paper
Abstract
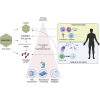
Immunogenicity has imposed a challenge to efficacy and safety evaluation of adeno-associated virus (AAV) vector-based gene therapies. Mild to severe adverse events observed in clinical development have been implicated with host immune responses against AAV gene therapies, resulting in comprehensive evaluation of immunogenicity during nonclinical and clinical studies mandated by health authorities. Immunogenicity of AAV gene therapies is complex due to the number of risk factors associated with product components and pre-existing immunity in human subjects. Different clinical mitigation strategies have been employed to alleviate treatment-induced or -boosted immunogenicity in order to achieve desired efficacy, reduce toxicity, or treat more patients who are seropositive to AAV vectors. In this review, the immunogenicity risk assessment, manifestation of immunogenicity and its impact in nonclinical and clinical studies, and various clinical mitigation strategies are summarized. Last, we present bioanalytical strategies, methodologies, and assay validation applied to appropriately monitor immunogenicity in AAV gene therapy-treated subjects.
Keywords: adeno-associated virus; bioanalytical methodologies and validation strategies; clinical mitigation; gene therapy; immunogenicity; nonclinical and clinical outcomes; risk assessment.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Hunt S.Y. Controversies in treatment approaches: gene therapy, IVF, stem cells, and pharmacogenomics. Nat. Educ. 2008;1:222.
Publication types
LinkOut - more resources
Full Text Sources