Validation of the COVID-19 IgG/IgM Rapid Test Cassette (BNCP - 402 and BNCP402) in a South African setting
- PMID: 36092371
- PMCID: PMC9452929
- DOI: 10.4102/sajid.v37i1.431
Validation of the COVID-19 IgG/IgM Rapid Test Cassette (BNCP - 402 and BNCP402) in a South African setting
Abstract
Background: Different diagnostic tools could improve early detection of coronavirus disease 2019 (COVID-19). A number of antibody-based serological point-of-care tests have been developed to supplement real-time reverse transcriptase polymerase chain reaction (RT-PCR)-based diagnosis. This study describes the validity of an antibody test, namely the immunoglobulin G (IgG)/immunoglobulin M (IgM) Rapid Test Cassette® (BNCP - 402 and BNCP402), manufactured by Spring Healthcare Services.
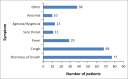
Methods: A prospective cohort validation study was undertaken at Chris Hani Baragwanath Academic Hospital between 16 July 2020 and 12 August 2020. A total of 101 patients admitted as COVID-19 cases under investigation were included in the study. They were divided into two categories depending on time since symptom onset: testing performed within seven days (early cohort) and after seven days (late cohort). The rapid antibody test was compared to the RT-PCR.
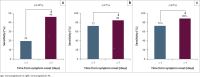
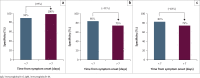
Results: Overall, the test has a sensitivity and specificity of 85.2% and 80.0%, respectively, for a combination of IgG and IgM. Sensitivity and specificity of IgG testing alone were 81.5% and 85%. Sensitivity improved for testing with increasing time from symptom onset; however, specifity was not significantly different.
Conclusion: The study data adds to the body of evidence that because of relatively low sensitivity and specificity, there is a limited role for antibody-based point-of-care testing in the acute phase of COVID-19 infection, as was the case with this IgG/IgM Rapid Test Cassette (BNCP - 402 and BNCP402). There may exist a role for such testing in patients recovered from prior COVID-19 infection or in seroprevalence studies; however, additional evaluations at later timepoints from symptom onset are required.
Keywords: COVID-19; South Africa; antibody test; immunoassay; point of care; rapid testing; sensitivity; serological test.
© 2022. The Authors.
Figures
References
-
- South African Department of Health . Minister Zweli Mkhize reports first case of Coronavirus Covid-19 [homepage on the Internet]. 2020. [cited 2020 Mar 5]. Available from: https://www.gov.za/speeches/health-reports-first-case-covid-19-coronavir...
LinkOut - more resources
Full Text Sources
