RNA structure mediated thermoregulation: What can we learn from plants?
- PMID: 36092413
- PMCID: PMC9450479
- DOI: 10.3389/fpls.2022.938570
RNA structure mediated thermoregulation: What can we learn from plants?
Abstract
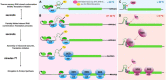
RNA molecules have the capacity to form a multitude of distinct secondary and tertiary structures, but only the most energetically favorable conformations are adopted at any given time. Formation of such structures strongly depends on the environment and consequently, these structures are highly dynamic and may refold as their surroundings change. Temperature is one of the most direct physical parameters that influence RNA structure dynamics, and in turn, thermosensitive RNA structures can be harnessed by a cell to perceive and respond to its temperature environment. Indeed, many thermosensitive RNA structures with biological function have been identified in prokaryotic organisms, but for a long time such structures remained elusive in eukaryotes. Recent discoveries, however, reveal that thermosensitive RNA structures are also found in plants, where they affect RNA stability, pre-mRNA splicing and translation efficiency in a temperature-dependent manner. In this minireview, we provide a short overview of thermosensitive RNA structures in prokaryotes and eukaryotes, highlight recent advances made in identifying such structures in plants and discuss their similarities and differences to established prokaryotic RNA thermosensors.
Keywords: RNA structure; plants; protein synthesis; temperature; thermosensor; translation.
Copyright © 2022 Thomas, Balcerowicz and Chung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

