Optimization of Arsenic Removal from Aqueous Solutions Using Amidoxime Resin Hosted by Mesoporous Silica
- PMID: 36092575
- PMCID: PMC9453956
- DOI: 10.1021/acsomega.2c03140
Optimization of Arsenic Removal from Aqueous Solutions Using Amidoxime Resin Hosted by Mesoporous Silica
Abstract
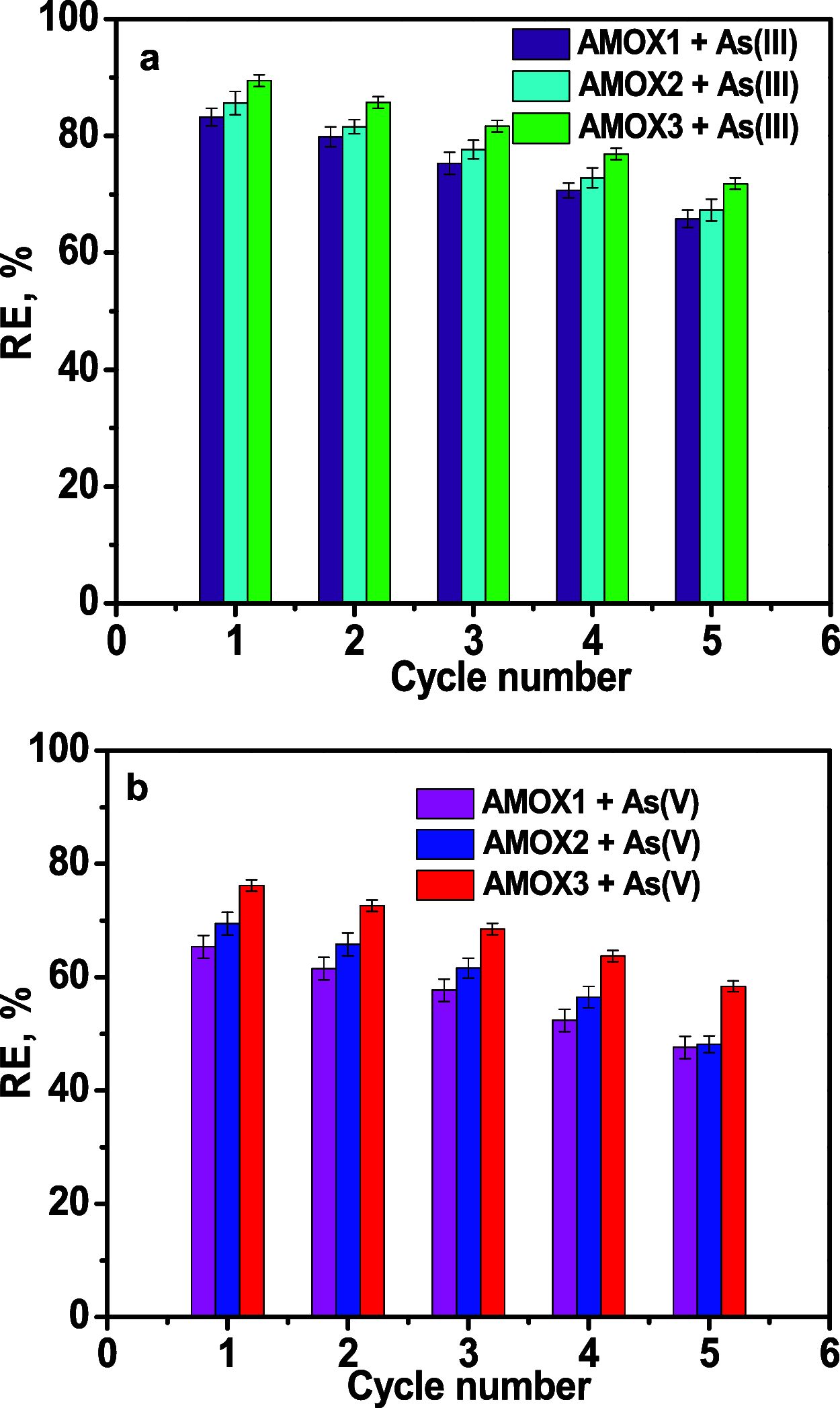
The paper reports on the performances of cross-linked amidoxime hosted into mesoporous silica (AMOX) in the removal of As(III) and As(V). The optimum pH for sorption of As(III) and As(V) was pH 8 and pH 5, respectively. The PFO kinetic model and the Sips isotherm fitted the best the experimental data. The thermodynamic parameters were evaluated using the equilibrium constant values given by the Sips isotherm at different temperatures and found that the adsorption process of As(III) and As(V) was spontaneous and endothermic on all AMOX sorbents. The spent AMOX sorbents could be easily regenerated with 0.2 mol/L HCl solution and reused up to five sorption/desorption cycles with an average decrease of the adsorption capacity of 18%. The adverse effect of the co-existing inorganic anions on the adsorption of As(III) and As(V) onto the sorbent with the highest sorption capacity (AMOX3) was arranged in the following order: H2PO4 - > HCO3 - > NO3 - > SO4 2-.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Yao R.; Yang H. An Overview on As(V) Removal from Water by Adsorption Technology. Ann. Muscoskel. Med. 2020, 4, 015–020. 10.17352/amm.000022. - DOI
-
- Ha H. N. N.; Phuong N. T. K.; An T. B.; Tho T. M.; Thang T. N.; Minh B. Q.; Du C. V. Arsenate Removal by Layered Double Hydroxides Embedded into Spherical Polymer Beads: Batch and Column Studies. J. Environ. Sci. Health, Part A: Environ. Sci. Eng. 2016, 51, 403–413. 10.1080/10934529.2015.1120526. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

