Emerging ROS-Modulating Technologies for Augmentation of the Wound Healing Process
- PMID: 36092613
- PMCID: PMC9453976
- DOI: 10.1021/acsomega.2c02675
Emerging ROS-Modulating Technologies for Augmentation of the Wound Healing Process
Abstract
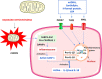
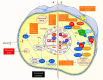
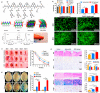
Reactive oxygen species (ROS) is considered a double-edged sword. The slightly elevated level of ROS helps in wound healing by inhibiting microbial infection. In contrast, excessive ROS levels in the wound site show deleterious effects on wound healing by extending the inflammation phase. Understanding the ROS-mediated molecular and biomolecular mechanisms and their effect on cellular homeostasis and inflammation thus substantially improves the possibility of exogenously augmenting and manipulating wound healing with the emerging antioxidant therapeutics. This review comprehensively delves into the relationship between ROS and critical phases of wound healing and the processes underpinning antioxidant therapies. The manuscript also discusses cutting-edge antioxidant therapeutics that act via ROS scavenging to enhance chronic wound healing.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Clark R. A.Wound repair. In The molecular and cellular biology of wound repair; Springer, 1988; pp 3–50.
-
- Homayouni-Tabrizi M.; Asoodeh A.; Abbaszadegan M. R.; Shahrokhabadi K.; Nakhaie Moghaddam M. An identified antioxidant peptide obtained from ostrich (Struthio camelus) egg white protein hydrolysate shows wound healing properties. Pharm. Biol. 2015, 53 (8), 1155–1162. 10.3109/13880209.2014.962061. - DOI - PubMed
-
- Trachootham D.; Lu W.; Ogasawara M. A.; Rivera-Del Valle N.; Huang P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10 (8), 1343–1374. 10.1089/ars.2007.1957. - DOI - PMC - PubMed
- Yin X.-M.; Dong Z.. Essentials of apoptosis-A guide for basic and clinical research; Humana Press, 2009.
Publication types
LinkOut - more resources
Full Text Sources

