Cation-Exchangeable Pralidoxime Chloride@bio-MOF-1 as a Treatment for Nerve Agent Poisoning and Sulfur Mustard Skin Poisoning in Animals
- PMID: 36092617
- PMCID: PMC9453934
- DOI: 10.1021/acsomega.2c01240
Cation-Exchangeable Pralidoxime Chloride@bio-MOF-1 as a Treatment for Nerve Agent Poisoning and Sulfur Mustard Skin Poisoning in Animals
Abstract
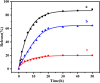
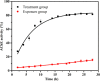
A 2-PAM@bio-MOF-1 composite was prepared by cationic exchange of counter N,N-dimethylammonium cations in the pores of the anionic, biocompatible metal-organic framework (bio-MOF-1) with pralidoxime chloride (2-PAM-Cl) by impregnation. In vitro drug release measurements revealed that the release rate of 2-PAM from 2-PAM@bio-MOF-1 in simulated body fluid (SBF) was more than four-fold higher than that in deionized water, indicating that the presence of endogenous cations in SBF triggered the release of 2-PAM through cation exchange. The release of 2-PAM was rapid within the first 10 h but was much slower over the period of 10-50 h. At room temperature, the maximum release rate of 2-PAM was 88.5% (15 mg of 2-PAM@bio-MOF-1 in 1 mL of SBF), indicating that the drug was efficiently released from the composite MOF in SBF. In simulated gastric fluid, 64.3% of 2-PAM was released from bio-MOF-1 into the simulated gastric fluid after 50h. This suggested that 2-PAM@bio-MOF-1 might be effective for enabling the slow release of 2-PAM in the human body. Indeed, the maximum reactivation rate of acetylcholinesterase in sarin-poisoned mice reached 82.5%. In addition, 2-PAM@bio-MOF-1 demonstrated the ability to adsorb and remove sulfur mustard (HD) in solution and from the skin of guinea pigs.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Loading and Sustained Release of Pralidoxime Chloride from Swellable MIL-88B(Fe) and Its Therapeutic Performance on Mice Poisoned by Neurotoxic Agents.Inorg Chem. 2022 Jan 24;61(3):1512-1520. doi: 10.1021/acs.inorgchem.1c03227. Epub 2021 Dec 30. Inorg Chem. 2022. PMID: 34969248
-
Penetrating the Blood-Brain Barrier for Targeted Treatment of Neurotoxicant Poisoning by Nanosustained-Released 2-PAM@VB1-MIL-101-NH2(Fe).ACS Appl Mater Interfaces. 2023 Mar 15;15(10):12631-12642. doi: 10.1021/acsami.2c18929. Epub 2023 Mar 3. ACS Appl Mater Interfaces. 2023. PMID: 36867458
-
Reduction by pyridostigmine pretreatment of the efficacy of atropine and 2-PAM treatment of sarin and VX poisoning in rodents.Fundam Appl Toxicol. 1992 Jan;18(1):102-6. doi: 10.1016/0272-0590(92)90201-r. Fundam Appl Toxicol. 1992. PMID: 1601200
-
Combining Two into One: A Dual-Function H5PV2Mo10O40@MOF-808 Composite as a Versatile Decontaminant for Sulfur Mustard and Soman.Inorg Chem. 2020 Aug 17;59(16):11595-11605. doi: 10.1021/acs.inorgchem.0c01392. Epub 2020 Jul 31. Inorg Chem. 2020. PMID: 32799468
-
Review of UV spectroscopic, chromatographic, and electrophoretic methods for the cholinesterase reactivating antidote pralidoxime (2-PAM).Drug Test Anal. 2012 Mar-Apr;4(3-4):179-93. doi: 10.1002/dta.327. Epub 2011 Sep 27. Drug Test Anal. 2012. PMID: 21953823 Review.
Cited by
-
Prospects, Advances, and Applications of BioMOF-Based Platforms.Top Curr Chem (Cham). 2025 Aug 27;383(3):34. doi: 10.1007/s41061-025-00512-0. Top Curr Chem (Cham). 2025. PMID: 40864352 Review.
References
-
- Beck J. M.Organophosphorus Nerve Agent Chemistry; Interactions of Chemical Warfare Agents and Their Therapeutics with Acetylcholinesterase. Ph.D. Thesis, The Ohio State University, Columbus, U. S., 2011.
-
- Noshad H.; Ansarin K.; Ardalan M. R.; Ghaffari A. R.; Safa J.; Nezami N. Respiratory Failure in Organophosphate Insecticide Poisoning. Saudi. Med. J. 2007, 28, 405–407. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

