Fingolimod versus interferon beta 1-a: Benefit-harm assessment approach based on TRANSFORMS individual patient data
- PMID: 36092642
- PMCID: PMC9459487
- DOI: 10.1177/20552173221117784
Fingolimod versus interferon beta 1-a: Benefit-harm assessment approach based on TRANSFORMS individual patient data
Abstract
Background: Fingolimod is a disease-modifying drug approved for multiple sclerosis but its benefit-harm balance has never been assessed compared to other active treatments.
Objectives: Our aim was to compare the benefits and harms of fingolimod with interferon beta-1a using individual patient data from TRial Assessing injectable interferon versus FTY720 Oral in RRMS trial.
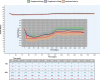
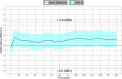
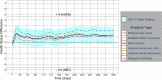
Methods: We modelled the health status of patients over time including Expanded Disability Status Scale measurements, relapses and any adverse events. We assessed the mean health status between arms and the proportion of patients whose health deteriorated or improved relatively to baseline, using a prespecified minimal important difference of 4.6. We performed sensitivity analyses to test our assumptions.
Results: Main and sensitivity analyses favoured fingolimod 0.5 mg over interferon beta-1a. The average health status difference was 1.01 (95% CI 0.93-1.08). Patients on fingolimod 0.5 mg were 0.47 (95% CI: 0.35-0.63, p < 0.001) times less likely to experience a relevant decline in health status compared to interferon beta-1a patients, with a number needed to treat of 7.10 [5.18, 11.23].
Conclusions: Fingolimod's net benefit over interferon beta-1a did not reach the clinical relevance over 1 year, but the decreased risk for health status deterioration may be more pronounced more long term and patients may prefer less treatment burden associated with fingolimod.
Keywords: Benefit–harm assessment; adverse events; fingolimod; health status; interferon beta-1a; multiple sclerosis.
© The Author(s), 2022.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jürg Kesselring was a member of the Data Safety Monitoring Board of Fingolimod studies from 2005 to 2017.
Figures
Similar articles
-
Benefit-harm balance of fingolimod in patients with MS: A modelling study based on FREEDOMS.Mult Scler Relat Disord. 2020 Nov;46:102464. doi: 10.1016/j.msard.2020.102464. Epub 2020 Aug 23. Mult Scler Relat Disord. 2020. PMID: 32889373 Clinical Trial.
-
Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States.J Med Econ. 2012;15(6):1088-96. doi: 10.3111/13696998.2012.693553. Epub 2012 May 24. J Med Econ. 2012. PMID: 22583065
-
Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study.Lancet Neurol. 2011 Jun;10(6):520-9. doi: 10.1016/S1474-4422(11)70099-0. Epub 2011 May 13. Lancet Neurol. 2011. PMID: 21571593 Clinical Trial.
-
Annualized relapse rate of first-line treatments for multiple sclerosis: a meta-analysis, including indirect comparisons versus fingolimod.Curr Med Res Opin. 2012 May;28(5):767-80. doi: 10.1185/03007995.2012.681637. Epub 2012 Apr 24. Curr Med Res Opin. 2012. PMID: 22462530
-
Fingolimod: a review of its use in relapsing-remitting multiple sclerosis.Drugs. 2014 Aug;74(12):1411-33. doi: 10.1007/s40265-014-0264-y. Drugs. 2014. PMID: 25063048 Review.
References
-
- FDA See FDA Adverse Event Reporting System (FAERS) Public Dashboard 2022. (search for fingolimod). Available at: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/overview
-
- NICE. Fingolimod for the treatment of highly active relapsing–remitting multiple sclerosis. 2012. Available from: https://www.nice.org.uk/guidance/ta254/chapter/4-Consideration-of-the-ev....
-
- FDA. MEDICATION GUIDE GILENYA®. 2019. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022527s029s030....
-
- Puhan MA, Singh S, Weiss COet al. et al. Evaluation of the benefits and Harms of aspirin for primary prevention of cardiovascular events: a comparison of quantitative approaches. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Nov. Report No.: 12(14)-EHC149-EF. AHRQ Methods for Effective Health Care. 2013. - PubMed
LinkOut - more resources
Full Text Sources

