Weight loss in a cardiovascular trial population identifies people at future risk of dementia
- PMID: 36092692
- PMCID: PMC9428278
- DOI: 10.1002/dad2.12352
Weight loss in a cardiovascular trial population identifies people at future risk of dementia
Abstract
Introduction: Populations at increased risk of dementia need to be identified for well-powered trials of preventive interventions. Weight loss, which often occurs in pre-clinical dementia, could identify a population at sufficiently high dementia risk.
Methods: In 12,975 survivors in the Heart Protection Study statin trial of people with, or at high risk of, cardiovascular disease, the association of weight change over 5 years during the trial with post-trial dementia recorded in electronic hospital admission and death records (n = 784) was assessed, after adjustment for age, sex, treatment allocation, and deprivation measures.
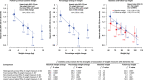
Results: Among the 60% without substantial weight gain (≤2 kg weight gain), each 1 kg weight loss was associated with a risk ratio for dementia of 1.04 (95% confidence interval, 1.02-1.07). Weight loss ≥4 kg and cognitive function below the mean identified participants aged ≥67 years with a 13% 10-year dementia risk.
Discussion: The combination of weight loss and high vascular risk identified individuals at high risk of dementia who could be recruited to dementia prevention trials.
Keywords: cognitive function; dementia; risk prediction; weight loss.
© 2022 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
All authors worked in the Clinical Trial Service Unit & Epidemiological Studies Unit of the Nuffield Department of Population Health at the University of Oxford. The Clinical Trial Service Unit & Epidemiological Studies Unit has a staff policy of not taking any personal payments directly or indirectly from industry (with reimbursement sought only for the costs of travel and accommodation to attend scientific meetings). Support for the present manuscript to all authors came from grants to the University of Oxford from Merck, Roche, the UK Medical Research Council, the British Heart Foundation and Cancer Research UK (see acknowledgements section for full details of funding). P.V., I.H., G.B., M.G., A.O., and M.N. have nothing further to disclose. W.W. reports support from the Chief Scientist's Office and the Alzheimer's Society, membership of a Data Safety Monitoring Board outside the area of this publication and a leadership or fiduciary role on the Stroke editorial board. R.B. reports he is chair of a NIHR funded trial (CHAPS) looking at compression hosiery to prevent post‐thrombotic syndrome (unpaid). R.C. is deputy chair of a not‐for‐profit Clinical Trial Company, Chief Executive of UK Biobank and chair of the Data Monitoring Committee of PROMINENT Data Safety Monitoring Board of Pemafibrate. J.A. reports a grant from Novartis and membership of Data Safety Monitoring Board (unpaid). M.M. reports grants from Novartis and Novo Nordisk. S.P. is statistician to the Data Monitoring Committee of the ORION‐4 trial of inclisiran. S.P. and R.C. are co‐inventors of a genetic test for statin‐related myopathy risk but receive no income from it. Author disclosures are available in the supporting information.
Figures


References
-
- World Health Organization. Dementia. 2021. Accessed May 5, 2022. Available from: https://www.who.int/news‐room/fact‐sheets/detail/dementia
-
- Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455‐532. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
