The Hepatitis C Care Cascade During the Direct-Acting Antiviral Era in a United States Commercially Insured Population
- PMID: 36092829
- PMCID: PMC9454032
- DOI: 10.1093/ofid/ofac445
The Hepatitis C Care Cascade During the Direct-Acting Antiviral Era in a United States Commercially Insured Population
Abstract
Background: Periodic surveillance of the hepatitis C virus (HCV) care cascade is important for tracking progress toward HCV elimination goals, identifying gaps in care, and prioritizing resource allocation. In the pre-direct-acting antiviral (DAA) era, it was estimated that 50% of HCV-infected individuals were diagnosed and that 16% had been prescribed interferon-based therapy. Since then, few studies utilizing nationally representative data from the DAA era have been conducted in the United States.
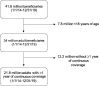
Methods: We performed a cross-sectional study to describe the HCV care cascade in the United States using the Optum de-identified Clinformatics® Data Mart Database to identify a nationally representative sample of commercially insured beneficiaries between January 1, 2014 and December 31, 2019. We estimated the number of HCV-viremic individuals in Optum based on national HCV prevalence estimates and determined the proportion who had: (1) recorded diagnosis of HCV infection, (2) recorded HCV diagnosis and underwent HCV RNA testing, (3) DAA treatment dispensed, and (4) assessment for cure.
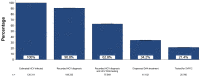
Results: Among 120,311 individuals estimated to have HCV viremia in Optum during the study period, 109,233 (90.8%; 95% CI, 90.6%-91.0%) had a recorded diagnosis of HCV infection, 75,549 (62.8%; 95% CI, 62.5%-63.1%) had a recorded diagnosis of HCV infection and underwent HCV RNA testing, 41,102 (34.2%; 95% CI, 33.9%-34.4%) were dispensed DAA treatment, and 25,760 (21.4%; 95% CI, 21.2%-21.6%) were assessed for cure.
Conclusions: Gaps remain between the delivery of HCV-related care and national treatment goals among commercially insured adults. Efforts are needed to increase HCV treatment among people diagnosed with chronic HCV infection to achieve national elimination goals.
Keywords: HIV/HCV coinfection; cascade of care; health claims database; hepatitis C elimination; hepatitis C monitoring.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

References
-
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol 2014; 61(1 Suppl):S58–68. - PubMed
-
- Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011; 9:509–16.e1. - PubMed
-
- Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet Lond Engl 2019; 393:1453–64. - PubMed

