NOD1 induces pyroptotic cell death to aggravate liver ischemia-reperfusion injury in mice
- PMID: 36092860
- PMCID: PMC9433815
- DOI: 10.1002/mco2.170
NOD1 induces pyroptotic cell death to aggravate liver ischemia-reperfusion injury in mice
Abstract
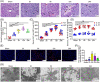
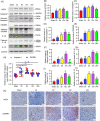
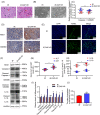
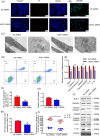
Nucleotide-binding oligomerization domain 1 (NOD1) can direct the release of inflammatory factors and influence autophagy and apoptosis in hepatic ischemia-reperfusion injury (IRI) in mice. As pyroptosis is involved in a number of inflammatory reactions, in this report, we investigated the potential for NOD1 to affect pyroptosis. We found that an increased expression of NOD1 during IRI was related to activation of the pyroptotic signaling pathway. With NOD1 activation, cleavage fragments of Caspase-1, gasdermin D (GSDMD), and interleukin (IL)-1β were all increased. Moreover, downregulation of NOD1 expression in AML12 cells exerted an opposite effect. Expression levels of cleaved-Caspase-1 and cleaved-GSDMD decreased after exposure to IRI and the number of cell membrane pores and apoptotic or pyroptotic cells decreased, along with the contents of inflammatory factors and lactate dehydrogenase in the supernatants of AML12 cells. Based on these findings, we conclude that NOD1 aggravates the pyroptotic cell death associated with hepatic ischemia-reperfusion injury in a mouse model via the Caspase-1/GSDMD axis. These findings help to alleviate pyroptotic cell death during liver transplantation or resection, providing new insights into novel protective therapies for liver IRI.
Keywords: NOD1; hepatocytes; ischemia‐reperfusion; pyroptosis.
© 2022 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
NOD1 activates autophagy to aggravate hepatic ischemia-reperfusion injury in mice.J Cell Biochem. 2019 Jun;120(6):10605-10612. doi: 10.1002/jcb.28349. Epub 2019 Jan 15. J Cell Biochem. 2019. PMID: 30644583
-
Macrophage Dvl2 deficiency promotes NOD1-Driven pyroptosis and exacerbates inflammatory liver injury.Redox Biol. 2025 Feb;79:103455. doi: 10.1016/j.redox.2024.103455. Epub 2024 Dec 4. Redox Biol. 2025. PMID: 39644526 Free PMC article.
-
Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia-reperfusion injury.Cell Death Dis. 2020 Apr 17;11(4):244. doi: 10.1038/s41419-020-2437-9. Cell Death Dis. 2020. PMID: 32303674 Free PMC article.
-
No longer married to inflammasome signaling: the diverse interacting pathways leading to pyroptotic cell death.Biochem J. 2022 May 27;479(10):1083-1102. doi: 10.1042/BCJ20210711. Biochem J. 2022. PMID: 35608339 Free PMC article. Review.
-
Structural Insight of Gasdermin Family Driving Pyroptotic Cell Death.Adv Exp Med Biol. 2019;1172:189-205. doi: 10.1007/978-981-13-9367-9_9. Adv Exp Med Biol. 2019. PMID: 31628657 Review.
Cited by
-
Programmed cell death, from liver Ischemia-Reperfusion injury perspective: An overview.Heliyon. 2024 Jun 17;10(13):e32480. doi: 10.1016/j.heliyon.2024.e32480. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040334 Free PMC article. Review.
-
Cell Death in Liver Disease and Liver Surgery.Biomedicines. 2024 Mar 1;12(3):559. doi: 10.3390/biomedicines12030559. Biomedicines. 2024. PMID: 38540172 Free PMC article. Review.
-
Gp78 deficiency in hepatocytes alleviates hepatic ischemia-reperfusion injury via suppressing ACSL4-mediated ferroptosis.Cell Death Dis. 2023 Dec 8;14(12):810. doi: 10.1038/s41419-023-06294-x. Cell Death Dis. 2023. PMID: 38065978 Free PMC article.
-
S-nitrosylated NEDD4 exacerbates gouty arthritis by upregulating NOD1 to induce pyroptosis.Genes Immun. 2025 Aug;26(4):365-375. doi: 10.1038/s41435-025-00341-7. Epub 2025 Jul 1. Genes Immun. 2025. PMID: 40594913
-
Role of the immune system in liver transplantation and its implications for therapeutic interventions.MedComm (2020). 2023 Dec 13;4(6):e444. doi: 10.1002/mco2.444. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38098611 Free PMC article. Review.
References
-
- Peralta C, Jiménez‐Castro MB, Gracia‐Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. Journal of Hepatology. 2013; 59(5): 1094‐1106. - PubMed
-
- Klune JR, Tsung A. Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. The Surgical Clinics of North America. 2010; 90(4): 665‐677. - PubMed
-
- Bahde R, Spiegel HU. Hepatic ischaemia‐reperfusion injury from bench to bedside. The British Journal of Surgery. 2010; 97(10): 1461‐1475. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
