Gut and obesity/metabolic disease: Focus on microbiota metabolites
- PMID: 36092861
- PMCID: PMC9437302
- DOI: 10.1002/mco2.171
Gut and obesity/metabolic disease: Focus on microbiota metabolites
Abstract
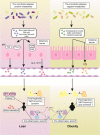
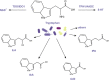
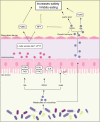
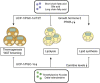
Obesity is often associated with the risk of chronic inflammation and other metabolic diseases, such as diabetes, cardiovascular disease, and cancer. The composition and activity of the gut microbiota play an important role in this process, affecting a range of physiological processes, such as nutrient absorption and energy metabolism. The active gut microbiota can produce a large number of physiologically active substances during the process of intestinal metabolism and reproduction, including short-chain/long-chain fatty acids, secondary bile acids, and tryptophan metabolites with beneficial effects on metabolism, as well as negative metabolites, including trimethylamine N-oxide, delta-valerobetaine, and imidazole propionate. How gut microbiota specifically affect and participate in metabolic and immune activities, especially the metabolites directly produced by gut microbiota, has attracted extensive attention. So far, some animal and human studies have shown that gut microbiota metabolites are correlated with host obesity, energy metabolism, and inflammation. Some pathways and mechanisms are slowly being discovered. Here, we will focus on the important metabolites of gut microbiota (beneficial and negative), and review their roles and mechanisms in obesity and related metabolic diseases, hoping to provide a new perspective for the treatment and remission of obesity and other metabolic diseases from the perspective of metabolites.
Keywords: gut microbiota; metabolic disease; metabolites; obesity.
© 2022 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures

References
-
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host‐bacterial mutualism in the human intestine. Science. 2005;307(5717):1915‐1920. - PubMed
-
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027‐1031. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
