Identification of a chromatin regulator signature and potential prognostic ability for adrenocortical carcinoma
- PMID: 36092868
- PMCID: PMC9459121
- DOI: 10.3389/fgene.2022.948353
Identification of a chromatin regulator signature and potential prognostic ability for adrenocortical carcinoma
Abstract
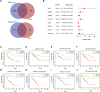
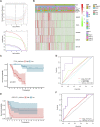
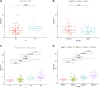
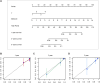
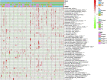
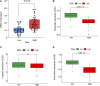
Objective: Adrenocortical carcinoma (ACC) is a rare malignant tumor. Chromatin regulators (CRs) can drive epigenetic changes, which have been considered as one of the most vital hallmarks of tumors. This study aimed to explore the CR signature for ACC in order to clarify the molecular basis of ACC's pathogenic mechanism and provide novel methods to diagnose and treat ACC clinically. Methods: This study obtained transcriptome sequencing datasets of ACC patients and sequencing data on normal adrenal tissues in TCGA and GTEx databases, respectively. Meanwhile, prognostic genes were selected through Lasso and Cox regression analyses. Using the transcriptome sequencing datasets of ACC patients downloaded from the GEO database to finish validation, we performed Kaplan-Meier (KM) analysis for evaluating the differential survival between low- and high-risk groups. Then, this work constructed the risk model for predicting ACC prognosis. TIMER 2.0 was employed to assess the differences in immune infiltration between the two groups. Furthermore, this work adopted the R package "pRRophetic" for exploring and estimating the sensitivity of patients to different chemotherapeutic agents. Results: A 5-CR model was established to predict ACC survival, and the CR signature was confirmed as a factor in order to independently predict ACC patient prognosis. In addition, a nomogram composed of the risk score and clinical T stage performed well in the prediction of patients' prognosis. Differentially expressed CRs (DECRs) were mostly associated with the cell cycle, base excision repair, colon cancer, gene duplication, homologous recombination, and other signaling pathways for the high-risk group. As for the low-risk group, DECRs were mainly enriched in allograft rejection, drug metabolism of cytochrome P450, metabolism of xenogeneic organisms by cytochrome P450, retinol metabolism, and other signaling pathways. According to TIMER analysis, the immune infiltration degrees of endothelial cells, M2 macrophages, myeloid dendritic cells, CD4+ Th1 cells, NKT cells, and M0 macrophages showed significant statistical differences between the high- and low-risk groups, and high infiltration levels of M0 and M2 macrophages were more pronounced in higher T stage (T3 and T4), N stage (N1), and clinical stages (III and IV). In addition, high-risk cases exhibited higher sensitivity to etoposide and doxorubicin. Additionally, low-risk patients had significantly decreased expression of RRM1 compared with high-risk cases, suggesting the better effect of mitotane treatment. Conclusion: This study identified the DECRs, which might be related to ACC genesis and progression. The pathways enriched by these DECRs were screened, and these DECRs were verified with excellent significance for estimating ACC survival. Drug sensitivity analysis also supported the current clinical treatment plan. Moreover, this study will provide reliable ideas and evidence for diagnosing and treating ACC in the clinic.
Keywords: adrenocortical carcinoma; chromatin regulator; diagnosis; prognosis; treatment.
Copyright © 2022 Li, Jia, Tang, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Identification of the ferroptosis regulator HELLS with prognostic value for adrenocortical carcinoma based on integrated analysis and experimental validation.Gland Surg. 2023 Sep 25;12(9):1251-1270. doi: 10.21037/gs-22-736. Epub 2023 Sep 15. Gland Surg. 2023. PMID: 37842529 Free PMC article.
-
Identification of a Ferroptosis-Related Signature Associated with Prognosis and Immune Infiltration in Adrenocortical Carcinoma.Int J Endocrinol. 2021 Jul 20;2021:4654302. doi: 10.1155/2021/4654302. eCollection 2021. Int J Endocrinol. 2021. PMID: 34335745 Free PMC article.
-
Identification and Verification of Immune-Related Genes Prognostic Signature Based on ssGSEA for Adrenocortical Carcinoma (ACC).Int J Gen Med. 2022 Feb 15;15:1471-1483. doi: 10.2147/IJGM.S345123. eCollection 2022. Int J Gen Med. 2022. PMID: 35210821 Free PMC article.
-
Current Issues in the Diagnosis and Management of Adrenocortical Carcinomas.2021 Aug 30. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. 2021 Aug 30. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 25905240 Free Books & Documents. Review.
-
Key MicroRNA's and Their Targetome in Adrenocortical Cancer.Cancers (Basel). 2020 Aug 6;12(8):2198. doi: 10.3390/cancers12082198. Cancers (Basel). 2020. PMID: 32781574 Free PMC article. Review.
Cited by
-
The Characteristics of Tumor Microenvironment Predict Survival and Response to Immunotherapy in Adrenocortical Carcinomas.Cells. 2023 Feb 27;12(5):755. doi: 10.3390/cells12050755. Cells. 2023. PMID: 36899891 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

