Flexible organic frameworks sequester neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo
- PMID: 36093029
- PMCID: PMC9384803
- DOI: 10.1039/d2sc02456j
Flexible organic frameworks sequester neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo
Abstract
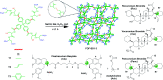
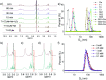
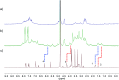
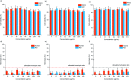
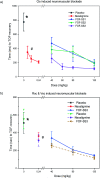
Supramolecular sequestration and reversal of neuromuscular block (NMB) have great clinical applications. Water-soluble flexible organic frameworks (FOFs) cross-linked by disulfide bonds are designed and prepared. Different linker lengths are introduced to FOFs to give them varied pore sizes. FOFs are anionic nanoscale polymers and capable of encapsulating cationic neuromuscular blocking agents (NMBAs), including rocuronium (Roc), vecuronium (Vec), pancuronium (Panc) and cisatracurium (Cis). A host-guest study confirms that FOFs bind NMBAs in water. The multivalency interaction between FOFs and NMBAs is able to sequester NMBAs, and prevent them from escaping. These FOFs are non-toxic and biocompatible. Animal studies show that FOFs are effective for the reversal of NMB induced by Roc, Vec and Cis, which shorten the time to a train-of-four ratio of 0.9 by 2.6, 3.8 and 5.7-fold compared to a placebo, respectively.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

References
LinkOut - more resources
Full Text Sources

