Heme oxygenase-1 mitigates liver injury and fibrosis via modulation of LNX1/Notch1 pathway in myeloid cells
- PMID: 36093061
- PMCID: PMC9450142
- DOI: 10.1016/j.isci.2022.104983
Heme oxygenase-1 mitigates liver injury and fibrosis via modulation of LNX1/Notch1 pathway in myeloid cells
Abstract
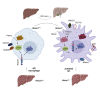
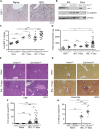
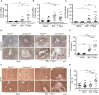
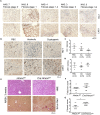
Activation of resident macrophages (Mϕ) and hepatic stellate cells is a key event in chronic liver injury. Mice with heme oxygenase-1 (HO-1; Hmox1)-deficient Mϕ (LysM-Cre:Hmox1 flfl ) exhibit increased inflammation, periportal ductular reaction, and liver fibrosis following bile duct ligation (BDL)-induced liver injury and increased pericellular fibrosis in NASH model. RiboTag-based RNA-sequencing profiling of hepatic HO-1-deficient Mϕ revealed dysregulation of multiple genes involved in lipid and amino acid metabolism, regulation of oxidative stress, and extracellular matrix turnover. Among these genes, ligand of numb-protein X1 (LNX1) expression is strongly suppressed in HO-1-deficient Mϕ. Importantly, HO-1 and LNX1 were expressed by hepatic Mϕ in human biliary and nonbiliary end-stage cirrhosis. We found that Notch1 expression, a downstream target of LNX1, was increased in LysM-Cre:Hmox1 flfl mice. In HO-1-deficient Mϕ treated with heme, transient overexpression of LNX1 drives M2-like Mϕ polarization. In summary, we identified LNX1/Notch1 pathway as a downstream target of HO-1 in liver fibrosis.
Keywords: Biological sciences; Cell biology; Human physiology; Stem cells research.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- An P., Wei L.L., Zhao S., Sverdlov D.Y., Vaid K.A., Miyamoto M., Kuramitsu K., Lai M., Popov Y.V. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020;11:2362. doi: 10.1038/s41467-020-16092-0. - DOI - PMC - PubMed
-
- Baisiwala S., Hall R.R., 3rd, Saathoff M.R., M Shireman J., Park C., Budhiraja S., Goel C., Warnke L., Hardiman C., Wang J.Y., et al. LNX1 modulates Notch1 signaling to promote expansion of the glioma stem cell population during temozolomide therapy in glioblastoma. Cancers. 2020;12:E3505. doi: 10.3390/cancers12123505. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

