This is a preprint.
A national audit of pancreatic enzyme prescribing in pancreatic cancer from 2015 to 2023 in England using OpenSAFELY-TPP
- PMID: 36093352
- PMCID: PMC9460968
- DOI: 10.1101/2022.07.08.22277317
A national audit of pancreatic enzyme prescribing in pancreatic cancer from 2015 to 2023 in England using OpenSAFELY-TPP
Update in
-
A National Audit of Pancreatic Enzyme Prescribing in Pancreatic Cancer from 2015 to 2023 in England Using OpenSAFELY-TPP.Semin Oncol Nurs. 2023 Jun;39(3):151439. doi: 10.1016/j.soncn.2023.151439. Epub 2023 May 2. Semin Oncol Nurs. 2023. PMID: 37142468 Free PMC article.
Abstract
Objectives: Cancer treatments were variably disrupted during the COVID-19 pandemic. UK guidelines recommend pancreatic enzyme replacement therapy (PERT) to all people with unresectable pancreatic cancer. The aim was to investigate the impact of COVID-19 on PERT prescribing to people with unresectable pancreatic cancer and to investigate the national and regional rates from January 2015 to January 2023.
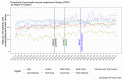
Data sources: With the approval of NHS England, we conducted this study using 24 million electronic healthcare records of people within the OpenSAFELY-TPP research platform. There were 22,860 people diagnosed with pancreatic cancer in the study cohort. We visualised the trends over time and modelled the effect of COVID-19 with the interrupted time series analysis.
Conclusions: In contrast to many other treatments, prescribing of PERT was not affected during the pandemic. Overall, since 2015, the rates increased steadily over time by 1% every year. The national rates ranged from 41% in 2015 to 48% in early 2023. There was substantial regional variation with the highest rates of 50% to 60% in West Midlands.
Implications for nursing practice: In pancreatic cancer, if PERT is prescribed, it is usually initiated in hospitals by clinical nurse specialists and continued after discharge by primary care. At just under 50% in early 2023, the rates were still below the recommended 100% standard. More research is needed to understand barriers to prescribing of PERT and geographic variation to improve quality of care. Prior work relied on manual audits. With OpenSAFELY, we developed an automated audit allowing for regular updates.
Conflict of interest statement
BG received research funding from the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, NHS England, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. BMK is employed as a pharmacist by NHS England and seconded to the Bennett Institute.
Figures


References
-
- Roberts KJ, Bannister CA, Schrem H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. Jan 2019;19(1):114–121. doi: 10.1016/j.pan.2018.10.010 - DOI - PubMed
-
- Sikkens EC, Cahen DL, van Eijck C, Kuipers EJ, Bruno MJ. The daily practice of pancreatic enzyme replacement therapy after pancreatic surgery: a northern European survey: enzyme replacement after surgery. J Gastrointest Surg. Aug 2012;16(8):1487–92. doi: 10.1007/s11605-012-1927-1 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
