Deep attention super-resolution of brain magnetic resonance images acquired under clinical protocols
- PMID: 36093418
- PMCID: PMC9458316
- DOI: 10.3389/fncom.2022.887633
Deep attention super-resolution of brain magnetic resonance images acquired under clinical protocols
Abstract
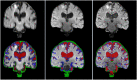
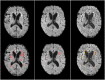
Vast quantities of Magnetic Resonance Images (MRI) are routinely acquired in clinical practice but, to speed up acquisition, these scans are typically of a quality that is sufficient for clinical diagnosis but sub-optimal for large-scale precision medicine, computational diagnostics, and large-scale neuroimaging collaborative research. Here, we present a critic-guided framework to upsample low-resolution (often 2D) MRI full scans to help overcome these limitations. We incorporate feature-importance and self-attention methods into our model to improve the interpretability of this study. We evaluate our framework on paired low- and high-resolution brain MRI structural full scans (i.e., T1-, T2-weighted, and FLAIR sequences are simultaneously input) obtained in clinical and research settings from scanners manufactured by Siemens, Phillips, and GE. We show that the upsampled MRIs are qualitatively faithful to the ground-truth high-quality scans (PSNR = 35.39; MAE = 3.78E-3; NMSE = 4.32E-10; SSIM = 0.9852; mean normal-appearing gray/white matter ratio intensity differences ranging from 0.0363 to 0.0784 for FLAIR, from 0.0010 to 0.0138 for T1-weighted and from 0.0156 to 0.074 for T2-weighted sequences). The automatic raw segmentation of tissues and lesions using the super-resolved images has fewer false positives and higher accuracy than those obtained from interpolated images in protocols represented with more than three sets in the training sample, making our approach a strong candidate for practical application in clinical and collaborative research.
Keywords: Magnetic Resonance Imaging; U-Net; brain imaging; deep learning; explainable artificial intelligence; generative adversarial networks; image reconstruction; super-resolution.
Copyright © 2022 Li, Castorina, Valdés Hernández, Clancy, Wiseman, Sakka, Storkey, Jaime Garcia, Cheng, Doubal, Thrippleton, Stringer and Wardlaw.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Bansal A., Ma S., Ramanan D., Sheikh Y. (2018). “Recycle-gan: unsupervised video retargeting,” in Proceedings of the European Conference on Computer Vision (ECCV) (Munich: ), 119–135.
-
- Bergstra J., Bengio Y. (2012). Random search for hyper-parameter optimization. J. Mach. Learn. Res. 13, 281–305.
-
- Bernal J., Valdés-Hernández M. d. C., Ballerini L., Escudero J., Jochems A. C., Clancy U., et al. (2020). “A framework for jointly assessing and reducing imaging artefacts automatically using texture analysis and total variation optimisation for improving perivascular spaces quantification in brain magnetic resonance imaging,” in Annual Conference on Medical Image Understanding and Analysis (Oxford: Springer; ), 171–183.
-
- Bernal J., Valdés-Hernández M. d. C., Escudero J., Armitage P. A., Makin S., et al. (2019). “Analysis of spatial spectral features of dynamic contrast-enhanced brain magnetic resonance images for studying small vessel disease,” in Annual Conference on Medical Image Understanding and Analysis (Liverpool: Springer; ), 282–293.
LinkOut - more resources
Full Text Sources

