Therapeutic arteriogenesis by factor-decorated fibrin matrices promotes wound healing in diabetic mice
- PMID: 36093431
- PMCID: PMC9452813
- DOI: 10.1177/20417314221119615
Therapeutic arteriogenesis by factor-decorated fibrin matrices promotes wound healing in diabetic mice
Abstract
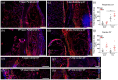
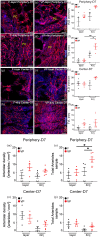
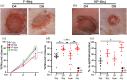
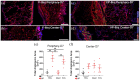
Chronic wounds in type-2 diabetic patients present areas of severe local skin ischemia despite mostly normal blood flow in deeper large arteries. Therefore, restoration of blood perfusion requires the opening of arterial connections from the deep vessels to the superficial skin layer, that is, arteriogenesis. Arteriogenesis is regulated differently from microvascular angiogenesis and is optimally stimulated by high doses of Vascular Endothelial Growth Factor-A (VEGF) together with Platelet-Derived Growth Factor-BB (PDGF-BB). Here we found that fibrin hydrogels decorated with engineered versions of VEGF and PDGF-BB proteins, to ensure protection from degradation and controlled delivery, efficiently accelerated wound closure in diabetic and obese db/db mice, promoting robust microvascular growth and a marked increase in feeding arterioles. Notably, targeting the arteriogenic factors to the intact arterio-venous networks in the dermis around the wound was more effective than the routine treatment of the inflamed wound bed. This approach is readily translatable to a clinical setting.
Keywords: PDGF-BB; VEGF; Wound-healing; angiogenesis; arteriogenesis; fibrin.
© The Author(s) 2022.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The fibrin gel immobilization scheme is the subject of patents upon which J.A.H. is named as inventor and has been licensed by a company in which J.A.H. is a shareholder.
Figures

References
-
- Saeedi P, Petersohn I, Salpea P, et al.. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2019; 157: 107843. - PubMed
-
- Zhang P, Lu J, Jing Y, et al.. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 2017; 49: 106–116. - PubMed
-
- Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017; 376: 2367–2375. - PubMed
-
- Saluja S, Anderson SG, Hambleton I, et al.. Foot ulceration and its association with mortality in diabetes mellitus: a meta-analysis. Diabet Med 2020; 37: 211–218. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

