Anlotinib improved the reactive cutaneous capillary endothelial proliferation induced by camrelizumab: a case report
- PMID: 36093549
- PMCID: PMC9459630
- DOI: 10.21037/tcr-22-426
Anlotinib improved the reactive cutaneous capillary endothelial proliferation induced by camrelizumab: a case report
Abstract
Background: Programmed cell death protein-1 (PD-1) or its ligand PD-L1 monoclonal antibodies, opening a new era of tumor immunotherapy, and they have significantly improved the overall survival of many patients with advanced solid tumors. However, in addition to its effectiveness, we should also pay attention to its adverse effects. The instructions of the PD-1 inhibitor camrelizumab clearly indicate that reactive cutaneous capillary endothelial proliferation (RCCEP) is the most common adverse reaction; it is common for many immune checkpoint inhibitors (ICIs). Here we describe a case that anlotinib improved RCCEP induced by anti-PD-1 blockade camrelizumab with some focus on further management of this symptoms.
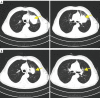
Case description: A 57-year-old man with squamous cell carcinoma of the upper lobe of the left lung, and with mediastinal lymphocyte and liver metastasis, received the fifth cycle of chemotherapy and immunotherapy with camrelizumab (200 mg, every 3 weeks). Four days after treatment with camrelizumab, the patient's face, head, neck, and chest skin had multiple scattered bright red round papules, which were diagnosed as RCCEP. The patient was treated with oral anlotinib (8 mg, once a day). After 5 days of treatment, the symptoms of RCCEP gradually eased, and the patient was discharged.
Conclusions: In conclusion, we have reported a case of RCCEP induced by anti-PD-1 blockade camrelizumab. The patient was given oral anlotinib to relieve the symptoms of RCCEP. Suggesting that anlotinib could be a potential management to reduce the adverse reactions who are treated with camrelizumab. The risk for RCCEP should always be kept in mind during camrelizumab treatment.
Keywords: Camrelizumab; anlotinib; case report; programmed cell death protein-1 (PD-1); reactive cutaneous capillary endothelial proliferation (RCCEP).
2022 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-426/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial.J Hematol Oncol. 2020 May 11;13(1):47. doi: 10.1186/s13045-020-00886-2. J Hematol Oncol. 2020. PMID: 32393323 Free PMC article. Clinical Trial.
-
A case report and literature review on reactive cutaneous capillary endothelial proliferation induced by camrelizumab in a nasopharyngeal carcinoma patient.Front Oncol. 2023 Nov 28;13:1280208. doi: 10.3389/fonc.2023.1280208. eCollection 2023. Front Oncol. 2023. PMID: 38090483 Free PMC article.
-
An epulis-like camrelizumab related reactive cutaneous capillary endothelial proliferation (RCCEP) in the oral cavity: A case report.Oral Oncol. 2023 May;140:106369. doi: 10.1016/j.oraloncology.2023.106369. Epub 2023 Mar 28. Oral Oncol. 2023. PMID: 36989963
-
Immune checkpoint inhibitor-related pneumonitis induced by camrelizumab: a case report and review of literature.Ann Palliat Med. 2021 Jul;10(7):8460-8466. doi: 10.21037/apm-21-23. Epub 2021 May 12. Ann Palliat Med. 2021. PMID: 34044548 Review.
-
The clinical application of camrelizumab on advanced hepatocellular carcinoma.Expert Rev Gastroenterol Hepatol. 2020 Nov;14(11):1017-1024. doi: 10.1080/17474124.2020.1807939. Epub 2020 Aug 18. Expert Rev Gastroenterol Hepatol. 2020. PMID: 32762583 Review.
Cited by
-
Observation on the Therapeutic Efficacy of Camrelizumab Combined with Chemotherapy in Non-small Cell Lung Cancer and the Cutaneous Immune-related Adverse Events: A Retrospective Study.Anticancer Agents Med Chem. 2025;25(8):574-587. doi: 10.2174/0118715206350978241105080452. Anticancer Agents Med Chem. 2025. PMID: 39781731
-
Immune checkpoint inhibitors combined with targeted therapy for long-term survival in advanced pulmonary squamous cell carcinoma after first-line failure: A case report and literature review.Medicine (Baltimore). 2025 Jun 20;104(25):e42724. doi: 10.1097/MD.0000000000042724. Medicine (Baltimore). 2025. PMID: 40550073 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
